

Original Article - Year 2011 - Volume 26 -
Correlation between electromyographic data and facial disability index in patients with long-term facial paralysis: implications for treatment outcomes
Correlação entre eletromiografia e índice de inabilidade facial em pacientes com paralisia facial de longa duração: implicações para o resultado de tratamentos
ABSTRACT
BACKGROUND: Several techniques are available for the assessment of facial movement and activity, and facial disability can be evaluated through self-administered questionnaires. However, the relationship between these objective and subjective measurements has not been examined to date. The present study examined the relationship between electromyographic data of the levator anguli oris muscle with the facial disability index in patients with long-term facial paralysis. We hypothesized that individuals with greater facial asymmetry have lower facial disability index scores.
METHODS: Patients were assessed using a clinical scale for the evaluation of facial expression, 2 facial disability index subscales, and the results of surface electromyography (sEMG). Seventeen long-term facial paralysis patients were analyzed and compared to 17 age- and gender-matched healthy controls.
RESULTS: Significant differences between right and left hemifaces during smiling and at rest were detected in the experimental group, but not in the controls. Statistical analyses also indicated a weak correlation between sEMG (facial asymmetry) and facial disability index.
CONCLUSIONS: The use of modern data analysis techniques such as sEMG in combination with self-reported data is of great benefit to clinicians and their patients. The identification of a combination of measurements from randomized trials that can best determine the most effective treatment for patients with facial paralysis should be the objective of future studies.
Keywords: Facial paralysis. Facial asymmetry. Quality of life. Electromyography.
RESUMO
INTRODUÇÃO: Apesar de o movimento facial e de a atividade muscular poderem ser quantificados por meio de diversas técnicas e de a inabilidade facial poder ser quantificada por meio de questionários de autoavaliação, a relação entre essas medidas objetivas e subjetivas ainda não foi investigada. O objetivo do presente trabalho foi correlacionar dados eletromiográficos dos músculos elevadores do ângulo da boca com o índice de inabilidade facial em pacientes com paralisia facial de longa duração. A hipótese do estudo foi de que indivíduos com maior assimetria facial apresentariam escores menores no índice de inabilidade facial.
MÉTODO: A avaliação consistiu na aplicação de uma escala clínica para avaliação da mímica facial, de duas subescalas do Índice de Inabilidade Facial e da realização do exame de eletromiografia de superfície (EMGs). Foram analisados 17 pacientes com paralisia facial de longa duração e os resultados foram comparados ao grupo controle, composto por 17 indivíduos saudáveis pareados por gênero e idade.
RESULTADOS: Os participantes do grupo pesquisa apresentaram diferenças significantes entre as hemifaces nas tarefas de repouso e sorriso. O mesmo não foi observado para os participantes do grupo controle. A análise estatística indicou correlação fraca entre os dados da EMGs (assimetria facial) e o Índice de Inabilidade Facial.
CONCLUSÕES: O uso de técnicas científicas modernas de análise de dados, como a EMGs, combinadas a medidas de autoavaliação oferece grandes possibilidades para clínicos e seus pacientes. A combinação de diferentes medidas em estudos randomizados que verifiquem o tipo de tratamento que oferece melhor resultado aos pacientes com paralisia facial deverá ser abordada em estudo futuro.
Palavras-chave: Paralisia facial. Assimetria facial. Qualidade de vida. Eletromiografia.
Apart from being associated with a wide range of functional and aesthetic problems, facial nerve paralysis commonly has negative emotional consequences. Facial nerve paralysis differs from other clinical conditions because a variety of underlying conditions can be the cause of this disability, such as cranial base trauma, congenital syndrome, cranial base tumors, and infectious diseases1,2. The incidence of Bell's Palsy, which is the most common cause of unilateral facial weakness, is approximately 25 in 100.0001. Although 84% of the cases show full recovery, 16% have chronic facial paralysis or paresis1. The psychological impact of facial disfigurement can result in fear of public areas and may adversely affect a patient's social life2,3.
According to evidence-based clinical practice guidelines, it is important to establish the benefits of assessing facial paralysis and treatment strategies with regard to function, aesthetic appearance, capacity for communication, or a combination of these parameters. Facial paralysis patients are usually examined through a subjective assessment of face functionality4, including the use of standardized photographic documentation and occasional video images2. As a general rule, a clinician's selection of a particular evaluation method is based on his or her training and expertise. In general, the clinical relevance and the effectiveness of the results are not taken into account.
The psychological and social difficulties faced by patients with facial paralysis have already been reported in the literature. Typical symptoms include alterations in emotional well-being, decreased self-esteem, anxiety, depression, and alternative behaviors such as social isolation and addiction2,5,6. In this sense, self-reported data are extremely important to support the assessment of facial neuromuscular dysfunction. Although the clinical recognition of the problems associated with facial paralysis has been described in the literature, there are few studies based on self-reporting as a means to evaluate treatment outcome7.
The main obstacle to the improvement of clinical care for facial paralysis patients is the development of an objective and quantitative method for the assessment of facial function. Surface electromyography (sEMG) can provide valuable information for clinicians because it provides data on the physiological processes that generate muscular strength, produce movement, and mediate the performance of several functions that enable our interaction with the world8.
The beneficial results of neuromuscular training in combination with sEMG have been reported in several studies5,9-12. The use of sEMG instruments in association with biofeedback techniques enables the provision of continuous information about physiological function such as muscular tension levels12. Neuromuscular training in combination with sEMG is based on the plasticity of the central nervous system. The brain is able to reorganize itself using visual and/or auditive information provided by sEMG11. The literature indicates that the therapeutic use of sEMG helps patients develop muscular control and decreases the incidence of synkinesis.
Although facial movement and muscular activity can be quantified by means of several techniques13-16, and facial disability can be quantified by self-reporting instruments17, the correlation between the results of objective and subjective assessment methods has not been described to date. The purpose of the present study was to determine the correlation between electromyographic data of the levator anguli oris muscle and the facial disability index in patients with long-term facial paralysis. We hypothesized that greater facial asymmetry would be correlated with lower scores on the facial disability index.
METHODS
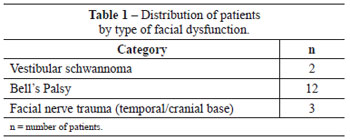
A total of 17 female patients with unilateral facial nerve paralysis who were aged between 35 and 60 years (average age, 42 years) were included in the study. All patients had static and/or dynamic facial asymmetry (Table 1). Patients with systemic or neuromuscular disease, cognitive loss, or asymmetry derived from craniofacial deformities were excluded from the research. An age-and gender-matched control group included 17 healthy volunteers.
All participants signed an informed consent form, and the study was approved by the ethics committee of the institution (CAPPesq HCFMUSP Nº 0201/08).
The inclusion criteria for the research group were as follows:
a) a medical diagnosis of long-term unilateral peripheral facial paralysis of more than 2 years from the appearance of symptoms; with or without previous surgical treatment (reconstruction or reanimation);
b) a score between 4 and 11 on the Facial Paralysis Clinical Assessment Scale7.
The inclusion criteria for the control group were as follows:
a) absence of a medical history of facial paralysis or trauma in the head and neck region;
b) a score between 19 and 20 on the Facial Paralysis Clinical Assessment Scale7.
Facial Paralysis Clinical Assessment Scale
Facial symmetry was assessed by two members of the staff. As previously described in the literature, the protocol includes the evaluation of voluntary facial movement on both sides independently and the assessment of involuntary movement, which are graded based on a scale.
The test is composed of three sections: strength of voluntary movement (raising eyebrows, closing eyelids, upper lip elevation, lateral traction of the upper lip, horizontal traction of the lip, lip closing, lowering the lower lip); strength of involuntary movement (eyelid closure during blinking, and during speaking and smiling); and presence of negative results (eyelid deformity at rest, lip deformity at rest, presence of synkinesis/spasms).
Movements are ranked as absent (0), partial (1), or integral (2). The negative findings receive negative scoring as follows: (0) absent, (-1) moderate, or (-2) pronounced. The sum of individual scores provides a final score that can have a maximum of 20 points.
sEMG Assessment
The elevators of the angle of the mouth (zygomaticus major and minor and levator anguli oris) were the muscular group analyzed in the study. This muscular group was selected because it is directly involved in smiling.
All sEMG assessments were performed using standardized surface electrodes (SDS500). The Miotool® 400 (Miotec Equipamentos Biomédicos, Porto Alegre, RS, Brasil) device with four channels was used in association with a computer system and double disposable electrodes (SDS500 Ag/AgCl, 10-mm diameter contact surfaces). This system enables the use of broadband filter systems, a 20-500 Hz band-pass filter, and a 60 Hz notch filter. The gain was set at 100 with low levels of noise (< 5 µV RMS), which is recommended by the International Society of Electrophysiological Kinesiology. The system uses active electrodes and a set of compact sensors that include a pre-amplifier, whose location with respect to the electrodes enables the reduction of artifacts and amplification of the electromyographic signal, which is transmitted to the connection cable (level of noise, <5 µV RMS).
All electromyographic signals were filtered, as previously described. Analysis of means, standard deviation, and minimum and maximum values of muscular activity during each task were evaluated using the software provided. Muscular activity was quantified in microvolts (µV).
Interelectrode distance was 10 mm. Two sets of bipolar electrodes were applied to the skin on each side of the face, over the levator anguli oris muscle to record electrical activity during voluntary smiling. A third electrode was placed on the right fist to act as a grounding electrode. The electric impedance in the areas of electrode placement was minimized by cleaning the skin with gauze soaked in 70% alcohol.
The baseline activity of the levator anguli oris muscle was recorded first followed by 3 voluntary smiling tests.
Step 1 - the participants were instructed to remain in a sitting position, with the head positioned in the Frankfurt horizontal plane. After the placement of electrodes on the skin, each participant was instructed to remain as still and relaxed as possible for one minute. Three independent recordings of 30 seconds each were obtained during resting. Step 2 - each participant was instructed to smile for 5 seconds and then keep the muscles relaxed for an additional 5 seconds, with 3 repetitions. During the tests, the participants were instructed to avoid sudden movements of the head. Electromyographic signal recording was started when the participants' muscle activity was at the baseline level.
Electromyographic Data Analysis
The analysis of the sEMG results was performed using the temporal domain assessment method. The information thus obtained includes when the event occurred and the amplitude (magnitude indicator of muscular activity) of its occurrence. Under resting conditions, the values obtained were the average root mean square (RMS) of the electromyographic activity observed during 30 seconds. The duration of muscular activity during dental pressure tasks (Al and MIC) was obtained by selecting the section of the trace that corresponded to muscle activation (on, peak, and off situation) using the electromyography software cursor and converting into microvolts.
The average action potential during movement was calculated using the software provided. To compare the results between participants, the sEMG amplitude values were normalized to the maximum contraction values recorded (percentage of maximum value for each electrode arrangement).
Electromyographic Data Reliability
Because the literature on the subject indicates subjectivity in the interpretation of sEMG readings, a reliability analysis was performed to determine the degree of agreement between examiners and thus validate the accuracy of the results. For this purpose, 30 electromyographic samples were randomly selected out of a total of 204. These samples were independently analyzed by two researchers with experience in the field. The correlation coefficient was high for all comparisons [confidence interval of 95% (IC 95%) 0.9788-0.9965], indicating good reliability between the examiners.
Facial Disability Index
The facial disability index is a self-reporting tool for the assessment of physical disability and psychosocial factors related to facial neuromuscular function17. Its objective is to provide information on the daily experience of patients who live with facial nerve disorder. This questionnaire has two sub-scales: the social well-being sub-scale (IBES), which includes items related to psychological and social aspects, and the physical function subscale (IFF), which assesses daily life activities (for example, tooth brushing, eating or drinking). This questionnaire was administered only to the experimental group.
Data Analysis
Statistical analyses were performed using Wilcoxon's test and Pearson's correlation coefficient, and significance was set at 0.05. The coefficient of asymmetry between both sides of the face and muscular contraction were calculated in both groups as follows:
research group - ratio between the non-paralyzed side/paralyzed side; control group - right side/left side.
RESULTS
sEMG
Differences were observed between the research and control groups. Descriptive statistics indicate that the research group showed lower electromyographic signal values during rest and smiling when compared to the control group. A greater variability in the recordings was observed during smiling in both groups (Table 2).

Comparison between the two groups revealed significant differences in the electromyographic signal values between the two sides of the face in the experimental group at rest (P = 0.049; Z = 1.96; T = 30) and during smiling (P < 0.001; Z = 3.62; T = 0). The coefficient of asymmetry was high during smiling (average at rest, 0.88 + 0.31; average during smiling, 3.69 + 5.53).
There were no significant differences between the sides of the face in the control group at rest (P = 0.57; Z = 0.57; T = 64.5) or during smiling (P = 0.29; Z = 1.07; T = 54). As observed in the experimental group, the control group also showed a high coefficient of asymmetry between both sides of the face during smiling (average at rest, 1.07 + 0.26; average during smiling, 1.3 + 0.67).
The differences in the facial asymmetry were verified by comparing the asymmetry coefficient between the groups under both conditions (Figure 1). The statistical analysis demonstrated a significant difference between the groups during smiling (P < 0.001; Z = 3.15; T = 10), indicating that the research group had a higher asymmetry index. The groups did not differ when compared during the resting conditions (P = 0.102; Z = 1.63; T = 42).

Figure 1 - Comparison between groups with regard to the asymmetry coefficient. IC = confidence interval; NP:P = ratio between non-paralyzed and paralyzed sides; R:L = ratio between right and left sides.
Facial Disability Index (IFF)
The facial disability scale was only applied to the experimental group. The average of IFF sub-scale values was 70 (minimum 50; maximum 95) and of IBES sub-scale was 68 (minimum 40; maximum 92).
Pearson's correlation coefficient was used to verify the association between sub-scale values and between sub-scale values and coefficients of asymmetry generated by sEMG. The results indicated a weak correlation between sub-scales and a very weak correlation between sub-scales and the coefficient of asymmetry (Table 3).

DISCUSSION
In the present study, the correlation between the results of electromyography of the levator anguli oris (smile) and the scores obtained from a self-reporting questionnaire (IFF) were analyzed in patients with long-term facial paralysis. The results showed no correlation between the objective examination (sEMG) and self-reporting measures, and our hypothesis was therefore not confirmed. Although there was a great degree of variation in muscular activity among the participants (sEMG), the results showed significant differences between the paralyzed and non-paralyzed sides of the face among patients with facial paralysis, which were not detected in healthy individuals. Reports showing muscular activity differences between the paralyzed and non-paralyzed sides of the face were previously published and indicate that facial nerve integrity is essential for the balance and symmetry of facial expression7,18.
Facial paralysis causes anatomical and physiological changes. Facial asymmetry may be caused not only by weaker muscle contractions on the paralyzed side, but also by hyperactivity of the non-paralyzed side2. Minor alterations tend to appear approximately 4 months after the lesion and include muscle contracture and muscular hypertrophy associated with synkinesis1. One of the limiting factors is the viability of the facial muscles because after 12 months without stimulation, the muscles degenerate and can quickly show signs of atrophy. In the present study, a 30% reduction in muscular activity during smiling was observed in the non-paralyzed side in the experimental group in comparison to the control group.
The follow-up of long-term facial paralysis patients is a controversial issue. The main reason for surgical and non-surgical treatments after facial nerve paralysis is to restore a patient's ability to smile1,3,5,7,12,19. Non-surgical treatment techniques include the application of botulinum toxin; physiotherapy; and stimulation/muscular training, such as therapy aimed at improving facial expression through electromyography and biofeedback, and specific facial exercises. However, even in well-designed studies, the results vary significantly likely due to the inherent difficulty in the accurate assessment of recovery2. If a consensus is reached on an effective procedure for the quantitative analysis of facial function and the comparison of global results in the follow-up of facial nerve lesions, the determination of training techniques may become clearer.
The literature indicates that the normal human face exhibits an asymmetry of 6% during the production of facial expressions20. This was observed through a combination of linear measures and invasive electromyography (needle electromyography) studies, and could therefore correspond to anatomical and muscular activity asymmetries caused by muscle imbalance and differences in measurements. These differences were not detected in the present study. The fact that sEMG is not as specific as invasive electromyography for the measurement of muscle activity likely explains the differences between the results of our study and those of others.
Although the literature indicates that IIF sub-scales are reliable with respect to the results of self-reporting in patients with facial nerve alterations5 and in enabling the monitoring of treatment outcomes7, this study did not detect a correlation between the sub-scales and objective measures. This fact must be further examined.
Effectiveness of treatment is a broad term that encompasses several questions related to treatment efficacy (What is the purpose of treatment?), treatment efficiency (Is one treatment better than the other?), and the effects of treatment (How does the treatment change behavior?). Objective and self-reporting measures should therefore be seen as complementary. The present study addresses the question of whether the perception of clinicians is the same as that of patients. This fact could potentially improve not only client satisfaction, but also the results of treatment. In this sense, there is a lack of evidence to determine the best techniques for soft tissue and neuromuscular reeducation1,10,11.
CONCLUSIONS
The use of modern data analysis techniques such as sEMG in combination with self-reporting tools is a promising approach for clinicians and their patients. The combination of different methods of analysis in randomized studies and the verification of the most beneficial therapeutic design for patients with facial paralysis will be the objective of future studies.
REFERENCES
1. Diels HJ. Facial paralysis: is there a role for a therapist? Facial Plast Surg. 2000;16(4):361-4.
2. Hadlock T. Facial paralysis: research and future directions. Facial Plast Surg. 2008;24(2):260-7.
3. Tate JR, Tollefson TT. Advances in facial reanimation. Curr Opin Otolaryngol Head Neck Surg. 2006;14(4):242-8.
4. Berg T, Jonsson L, Engström M. Agreement between the Sunnybrook, House-Brackmann, and Yanagihara facial nerve grading systems in Bell's palsy. Otol Neurotol. 2004;25(6):1020-6.
5. VanSwearingen JM, Brach JS. Changes in facial movement and synkinesis with facial neuromuscular reeducation. Plastic Reconstr Surg. 2003; 111(7):2370-5.
6. Finn JC, Cox SE, Earl ML. Social implications of hyperfunctional facial lines. Dermatol Surg. 2003;29(5):450-5.
7. Salles AG, Toledo PN, Ferreira MC. Botulinum toxin injection in long-standing facial paralysis patients: improvement of facial symmetry observed up to 6 months. Aesthetic Plast Surg. 2009;33(4):582-90.
8. De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13(2):135-63.
9. Segal B, Zompa I, Danys I, Black M, Shapiro M, Melmed C, et al. Symmetry and synkinesis during rehabilitation of unilateral facial paralysis. J Otolaryngol. 1995;24(3):143-8.
10. Brach JS, VanSwearingen JM, Lenert J, Jonhson PC. Facial neuromuscular retraining for oral synkinesis. Plast Reconstr Surg. 1997;99(7):1922-31.
11. Cronin GW, Steenerson RL. The effectiveness of neuromuscular facial retraining combined with electromyography in facial paralysis rehabilitation. Otolaryngol Head Neck Surg. 2003;128(4):534-8.
12. Vaiman M, Shlamkovich N, Kessler A, Eviatar E, Segal S. Biofeedback training of nasal muscles using internal and external surface electromyography of the nose. Am J Otolaryngol. 2005;26(5):302-7.
13. Linstrom CJ. Objective facial motion analysis in patients with facial nerve dysfunction. Laryngoscope. 2002;112(7 Pt 1):1129-47.
14. Kang TS, Vrabec JT, Giddings N, Terris DJ. Facial nerve grading systems (1985-2002): beyond the House-Brackmann scale. Otol Neurotol. 2002;23(5):767-71.
15. Mehta RP, Zhang S, Hadlock TA. Novel 3-D video for quantification of facial movement. Otolaryngol Head Neck Surg. 2008;138(4):468-72.
16. Linstrom CJ, Silverman CA, Susman WM. Facial-motion analysis with a video and computer system: a preliminary report. Am J Otol. 2000;21(1):123-9.
17. VanSwearingen JM, Brach JS. The facial disability index: reliability and validity of a disability assessment instrument for disorders of the facial neuromuscular system. Phys Ther. 1996;76(12):1288-98.
18. Deleyiannis FW, Askari M, Schmidt KL, Henkelmann TC, VanSwearingen JM, Manders EK. Muscle activity in the partially paralyzed face after placement of a fascial sling: preliminary report. Ann Plast Surg. 2005;55(5):449-55.
19. Coulson SE, Adams RD, O'Dwyer NJ, Croxson GR. Physiotherapy rehabilitation of the smile after long-term facial nerve palsy using video self-modeling and implementation intentions. Otolaryngol Head Neck Surg. 2006;134(1):48-55.
20. Burres SA. Facial biomechanics: the standards of normal. Laryngoscope. 1985;95(6):708-14.
1. Doctor of Sciences from the School of Medicine of Universidade de São Paulo (FMUSP), speech therapist of the Department of Physiotherapy, Speech Therapy and Occupational Therapy of the FMUSP, São Paulo, SP, Brazil.
2. Doctor of Sciences from FMUSP, post-doctoral student at the Department of Physiotherapy, Speech Therapy and Occupational Therapy of FMUSP, São Paulo, SP, Brazil.
3. Master of Sciences from FMUSP, technical assistant of the Support Unit in Speech Therapy at the Central Institute of Hospital das Clínicas da FMUSP, São Paulo, SP, Brazil.
4. Full professor at the Department of Surgery of FMUSP, associate professor at the Division of Plastic Surgery of the Department of Surgery of FMUSP, São Paulo, SP, Brazil.
5. Full professor at the Department of Physiotherapy, Speech therapy and Occupational Therapy of FMUSP, São Paulo, SP, Brazil.
Correspondence to:
Claudia Regina Furquim de Andrade
Rua Cipotânea, 51 - Cidade Universitária
São Paulo, SP, Brazil - CEP 05360-160
E-mail: clauan@usp.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Paper received: September 13, 2011
Paper accepted: October 25, 2011
Study conducted with FAPESP support, through scholarship (Process No 2008/02687-5).
Study conducted at the Speech Therapy Support Unit of the Central Institute of Hospital das Clínicas da Universidade de São Paulo and Plastic Surgery Department of Hospital das Clínicas of the School of Medicine of Universidade de São Paulo, São Paulo, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter