

Original Article - Year 2011 - Volume 26 -
Replacement of fetal calf serum by human serum as supplementation for human fibroblast culture
Substituição de soro bovino fetal por soro humano como suplemento para cultura de fibroblastos humanos
ABSTRACT
Background: Fetal calf serum (FCS) is commonly used as supplement in the culture medium for fibroblast cell cultures. This form of supplementation is far from ideal, as samples quality varies from batch to batch and its composition is not completely known. FCS may contain virus and prion contamination and it may also cause immunologic complications to humans. Due to those facts, a worldwide effort is being made to find alternatives to the use of xenobiotic elements in cell cultures. Human serum would be a safer alternative for FCS use, providing maintenance for cell in clinical appliance. Methods: We assayed human serum as a replacement of FCS in human fibroblasts culture. Human serum was obtained from blood of 10 healthy volunteers, submitted to serological evaluation. Fibroblasts were cultivated in multiwell plates containing either Dulbecco's Modified Eagle's Medium (DMEM) plus 10% FCS (D10) or DMEM plus 10% human serum (D10H). After 24 to 264 hours cells were counted and results were expressed in mean ± standard error of the mean to obtain cell proliferation curves. Results: There was no statistical difference between both proliferation groups. Human serum supported growth and proliferation of human fibroblasts, with a potential as substitute for FCS in cell culture. Cell morphology was different in human serum presence appearing to be smaller and rounded as compared to cells kept in D10. Conclusions: These results allow us to infer that human serum can substitute FCS in fibroblasts cell culture, and that fibroblasts cultured in human serum present morphology similar to in vivo fibroblasts.
Keywords: Serum. Cell culture techniques. Cells, cultured. Fibroblasts. Cell proliferation.
RESUMO
Introdução: Soro bovino fetal (SBF) é comumente usado como suplemento no meio de cultura para cultivar fibroblastos. Essa forma de suplementação, porém, não é ideal, pois a qualidade das amostras de SBF é variada e sua composição não é completamente conhecida. Além disso, o SBF pode apresentar contaminação por vírus e príons ou causar complicações imunológicas. Assim, a comunidade científica tem buscado alternativas ao uso de elementos xenobióticos em cultura celular. O soro humano pode ser uma dessas alternativas, principalmente para aplicação clínica. Métodos: Soro humano, obtido de sangue de 10 voluntários saudáveis submetidos a avaliação sorológica prévia, foi testado como substituto do SBF em cultura de fibroblastos humanos. As células foram cultivadas em placas multipoços, contendo Dulbecco's Modified Eagle's Medium (DMEM) mais 10% de SBF (D10) ou DMEM mais 10% de soro humano (D10H). Entre 24 e 264 horas de exposição aos meios testados, as células foram contadas e os resultados foram expressos em média ± erro padrão da média, para obtenção de curvas de proliferação celular. Resultados: Não houve diferença estatística entre os grupos de proliferação. Fibroblastos na presença de soro humano aparentavam ser menores e mais arredondados em comparação àqueles mantidos em D10. Conclusões: Os resultados permitem inferir que o soro humano pode substituir o SBF em cultura de fibroblastos e que fibroblastos cultivados em meio suplementado por soro humano apresentam morfologia mais semelhante àqueles in vivo.
Palavras-chave: Soro. Técnicas de cultura de células. Células cultivadas. Fibroblastos. Proliferação celular.
Since the beginning of the twentieth century, scientists have searched for methods to isolate and grow skin and/or skin cells for research and clinical application1. Currently, liquid or gel cell culture is a fundamental tool for research and for the growth of cells for therapeutic use2. For cells to survive and grow in vitro, special combinations of nutrients are required to be present in culture media to provide the optimal conditions for different cell types3. The medium used for fibroblast cultivation is universally supplemented with fetal calf serum (FCS), a complex mixture containing growth factors that are necessary to maintain cellular function and permit proliferation4.
FCS is easily obtained and contains a high concentration of growth factors and a low concentration of gamma globulins, compared to other sera originating from animals. This fact contributed to the adoption of FCS as the standard supplement for cell culture media5.
Normally, FCS is used to supplement the culture medium at a concentration of 5% to 20%. A thousand different components are present in its composition, of which only approximately 200 have been defined. These components include hormones, vitamins, nucleosides, amino acids, lipids, transport proteins (albumin, globin, transferrin), binding factors (fibronectin and laminin), stabilizing factors, detoxifying agents, proliferation factors, and growth factors6.
This form of supplementation is not ideal because the component concentrations can change from batch to batch and the full composition of FCS is not known. Further, FCS may contain pathogenic viruses or prions. Additionally, immunological complications may occur since FCS contains proteins that induce immune reactions7,8.
FCS leads to increased DNA synthesis in spleen cells of healthy rats9 and increases the formation of B-lymphocyte plaques in rats, leading to increased DNA and IgM synthesis in these cells10. FCS also induces a blastogenic response in peripheral blood lymphocytes11, and its presence in human tumor cell culture media may be responsible for antigenic alterations of these cells' membranes12. FCS has also been demonstrated to induce nonspecific cytotoxic responses in human lymphocytes, an occurrence which does not happen when the cells are grown in media supplemented with human serum. Both of these responses in cultured human cells were shown to be dose-dependent and triggered by a single exposure13.
Some studies have shown that patients undergoing cardiomyoplasty, using myofibroblasts grown in the presence of FCS, developed ventricular arrhythmias and suffered sudden death14. Burn patients who received transplants of in vitro cultivated keratinocytes have developed immune responses, which led to rejection of the graft15.
It is important to emphasize that FCS is classified as a low risk product for the transmission of bovine spongiform encephalopathy (BSE) since the prion that causes this disease cannot cross the placental barrier. The BSE prion has a tropism for the central nervous system of animals and is rarely found in the blood. However, many other diseases can be transmitted through serum, restricting the use of sera derived from animals in areas where such diseases are endemic. Thus, according to international trade rules, FCS must be obtained from areas which are free of cattle diseases; however, quality control tools are relatively primitive and expensive16.
Currently, there is a worldwide effort to find alternatives for the use of xenobiotic elements in cell culture media5,17,18. The utility of human serum in promoting cell growth was described by Chang19 following a lengthy study of human epithelial cells cultured in the presence of human serum. These studies demonstrated that sera from different individuals differentially impacted the growth rate of epithelial cells. Other studies with human cells, including conjunctival epithelial cells20, chondrocytes21, and bone marrow cells22, have demonstrated that human serum is capable of supporting the growth and normal phenotype of these cells. Indeed, these studies demonstrated that the cells had a higher rate of multiplication in media supplemented with human serum as compared to that supplemented with FCS23. Tallheden et al.24 demonstrated that human serum has higher levels of growth factors derived from the epidermis and platelets as compared to FCS.
Based on this knowledge, the present research was designed to examine replacing FCS with human serum as a culture enrichment factor. The cells chosen for this initial study were dermal fibroblasts - cells which have a key role in the process of wound contraction. Thus, this paper presents the results of an investigation into the substitution of FCS by human serum in culture media and its impact on promoting the in vitro proliferation of human fibroblasts.
METHODS
This study was approved by the Ethics Committee for Analysis of Research Projects (CAPPesq), Clinics Hospital of the Medical College of the University of São Paulo (FMUSP), number 2010 (01/23/2008), and was conducted in the Research Cell Culture and Wound Healing Laboratory within the Department of Plastic Surgery of FMUSP.
Acquisition of Human Serum
Human serum, 10-20 mL (mean = 15.7 ± 3.9 mL), was recovered from blood samples (400 mL) obtained from 10 healthy adult volunteers (both sexes), submitted for the following serological assessments: HIV, hepatitis B, hepatitis C, syphilis, Chagas disease, HTLV I and II, cytomegalovirus, and toxoplasmosis. The volunteers were informed about the purpose and consequences of the study and then signed an informed consent form. The blood samples were collected by venipuncture, performed at the Blood Bank of the Central Institute of HCFMUSP. The blood samples were sent to the laboratory where the serum was separated by centrifugation (10,000 rpm for 20 minutes).
Study Groups
Cell culture medium containing 10% human serum (D10H) was produced by adding an appropriate volume of pooled human serum to Dulbecco's modified Eagle's medium (DMEM; GIBCO 11965.092, Life Technologies, Baltimore, MD, USA) containing 2 mM glutamine (GIBCO 25.030.081, Life Technologies), 100 U/mL penicillin, 100 µg/mL streptomycin, and 25 µg/mL amphotericin B (Antibiotic Antimycotic; GIBCO 15249.062, Life Technologies). This medium was filtered to remove any contaminants and pathogens acquired during the preparation, minimizing the potential risk of loss of growth factors.
Medium supplemented with 10% FCS (D10) was identically prepared except that the human serum was replaced with FCS (GIBCO 16000.044, Life Technologies).
Fibroblast Cultures
Using the explant method, primary cultures of fibroblasts were initiated from full-thickness human skin (n = 4) derived from cosmetic mastoplasty procedures. The explants were cultivated in culture flasks with 25 cm2 surface areas (90025 TPP) containing D10H or D10 at 37°C in a humidified, 5% carbon dioxide (CO2) atmosphere. The culture medium was changed every 72 hours. The macroscopic behavior of the skin fragments was observed over time. These primary cultures of fibroblasts reached subconfluence after about four weeks of growth and, subsequently, were trypsinized and subcultured in the same medium. Thereafter, the cells were subcultured at weekly intervals.
Fibroblast Proliferation
Fibroblast growth assays were conducted using nonconfluent, asynchronous cultures. This was accomplished by dispensing 1-mL volumes of culture medium, containing1.5 x 103 freshly trypsinized fibroblasts, into triplicate wells of 9.62 cm2 (6 multiwell plates; Falcon 35 3047). Cultures were incubated for 11 days. After 24, 48, 72, 96, 120, and 264 hours, the fibroblasts were trypsinized and the number of released cells were counted using a Neubauer hemocytometer; cell viability was assessed by dye exclusion using 0.4% trypan blue (Sigma T-8154). The tests were performed on triplicate cultures with the number of cells from each culture being counted twice; values were expressed as the number of cells per well ± standard error of the mean (SEM).
Statistics
Cohort or follow-up studies for independent samples were performed; P < 0.05 was considered significant.
RESULTS
Macroscopic Aspects of the Cultures
Skin fragments demonstrated greater difficulty adhering to the flask surface in the presence of D10H as compared to D10 (Figure 1).

Figure 1 - Primary culture in 25-cm2 surface flasks one week after explanting. On the right, three bottles containing D10H and on the left, three bottles containing D10. A lower adherence of skin fragments in the presence of human serum as compared to the medium with fetal calf serum was observed.
Morphology of Dermal Fibroblasts
Observed with an inverted light microscope, skin explants in D10H-containing media took one week longer to release fibroblasts when compared to explants cultured in media with D10. Cells in D10H-containing media were more elongated and presented more intracytoplasmic granules than those maintained in D10-containing media (Figure 2). After several subcultures, the fibroblasts changed their phenotype, becoming more rounded in the presence of D10H than those cultured with D10 (Figure 3).
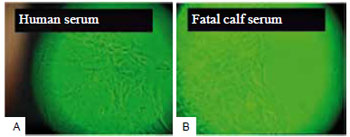
Figure 2 - Cultured fibroblasts at two weeks. In the presence of human serum (A), the cells were more elongated and presented larger number of intracytoplasmic granules, as compared to fibroblasts grown in fetal calf serum (B). In both cases, keratinocytes are present among the fibroblasts (OM - 10x).

Figure 3 - Details of cell culture after three weeks. Breakdown in the organization and small cell volume were observed among the fibroblasts grown in the presence of human serum (A and C) as compared to fibroblasts grown in fetal bovine serum (B and D). Fibroblasts cultured in the presence of human serum demonstrated a more rounded morphology (E) as compared to the cells grown with fetal calf serum (F) (OM - 10x). Number of cells ± standard error of the mean (SEM); P < 0.001.
Fibroblast Proliferation Assay
Fibroblast proliferation was observed throughout the experiments. After 48 hours, there were 6,565 ± 590.83 fibroblasts in the medium with human serum and 6,041.5 ± 216.81 in the medium with FCS. After 11 days, the number of cells was 99,791.5 ± 24,793.93 in D10H media and 68,645.75 ± 22,452.39 in D10 media (Figure 4).

Figure 4 - Comparison of the proliferation rate of human fibroblasts (n = 4) cultured in the presence of fetal calf serum or human serum over time (hours). SEM = standard error of the mean; FCS = fetal calf serum; HS = human serum.
DISCUSSION
FCS is the most widely used animal serum supplement for cell culture media because it is easily available, is easily stored, and contains high concentrations of growth factors. However, this supplement is far from ideal because its nutrient concentration varies from batch to batch, its composition is not fully defined, and it may be contaminated with viruses or prions7,8. For clinical applications, it would be safer to avoid using xenobiotic elements in human cell culture.
The use of human blood elements in cell culture has shown promising results, which has encouraged researchers to replace FCS in in vitro cultures of human fibroblasts by a similar nutrient derived from humans. Kurita et al.25 compared fibroblast growth in media supplemented with three human blood derivatives: serum, platelet-rich plasma, and platelet-poor plasma. They observed that serum and platelet-rich plasma stimulated intensive fibroblast growth, with results similar to those observed in FCS-supplemented media; the proliferation rate was significantly lower using platelet-poor plasma. These findings correlate with the presence of growth factors in platelets, such as platelet-derived growth factor that is known to be mitogenic for fibroblasts26. They also observed that the ability to stimulate cell proliferation varied somewhat between plasma or serum samples obtained from different individuals, but the variation was not linked to donor gender or age.
Remarkably, in this study, we observed that adhesion of skin fragments to the surface of the culture flask was delayed in the presence of human serum (Figure 1). Since this result was not yet reported, further studies are necessary to confirm and clarify this finding. Phenotypic differences between in vivo and in vitro grown fibroblasts have previously been reported. One such observation was that monolayers of fibroblasts cultured in the presence of FCS presented as wider cells as compared to the more rounded cells found, in vivo, in extracellular matrix27. Similarly, we also observed morphological differences between fibroblasts grown in the presence of FCS and those grown in human serum-supplemented media. Cultured fibroblasts showed a more rounded morphology when exposed to D10H medium than when grown in D10, suggesting that the presence of human serum is responsible for the in vivo phenotype (Figures 2 and 3).
However, this delayed adhesion was not observed to have an impact on the proliferation rate of these cells; the proliferation rate of the fibroblasts was the same regardless of whether the cells were grown in D10H or D10 (Figure 4), as previously reported by Cooper & Goldstein28. However, these authors also reported that the number of rounds of replication that occurred over the lifespan of the cultured cells was significantly reduced in the presence of human serum.
Mazlyzam et al.29 analyzed the lifespan and protein synthesis of cultured fibroblasts in 10% human serumsupplemented media compared to that of cells cultured in 10% FCS-supplemented medium. The authors observed that cellular proliferation was faster in the human serumcontaining media, leading to shorter duration of cellular duplication and maintenance of the physiological phenotype of the fibroblasts. However, this increased cellular proliferation rate was not observed in the present study, possibly due to the difference in the composition of the medias used in the two studies. Mazlyzam et al.29 used Ham's F12 and DMEM supplemented with 10% FCS, while in our study we did not include Ham's F12 in the media.
According to the Mazlyzam et al.29 report, the level of type I collagen gene expression was maintained in cultured fibroblasts with an increase of its expression in early passages and up-regulation of genes related to the expression of type III collagen. This is important for the down-regulation of gene expression of alpha-smooth muscle actin and of extracellular proteins involved in wound contraction and cicatrix formation29.
Kondo et al.30 obtained human fetal skin fibroblasts and compared in vitro cell migration in culture media containing either human serum or FCS. In 48% of the samples, human serum inhibited cell migration and maintained higher levels of proliferation. These authors also observed that the inhibition was dependent on the human serum concentration and on the age of the serum donor, with serum from older donors yielding a more pronounced inhibition of migration31. This inhibition may be attributed to an interference with collagen synthesis, but the authors also raised the hypothesis that inhibition would result from the levels of alpha 2-macroglobulin and low-density lipoprotein.
We are performing other studies to examine the possibility of substituting other human blood components, such as a platelet lysate, for FCS. We intend to compare the results obtained from the new studies with those obtained by other authors. The ultimate aim of this work is to produce humanized skin that can be used clinically in the treatment of chronic ulcers of the lower limb. This next phase of work will be conducted at the Cellular Therapy Laboratory of the Central Institute of the Clinics Hospital.
CONCLUSION
The results obtained in this study indicate that human serum can substitute for FCS in human fibroblast culture medium. This substitution is especially important for clinical application because it minimizes the possibility of immunological complications related to the use of xenobiotic components.
REFERENCES
1. Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305-13.
2. Gruber DF, Jayme DW. Cell and tissue culture media: history and terminology. In: Cell biology: a laboratory handbook. New York: Academic Press; 1994. p. 45-50.
3. Madigan MT, Martinko JM, Stahl DA, Clark DP. Brock biology of microorganisms. 12nd ed. Upper Saddle River: Prentice Hall; 2008. p. 943.
4. Eagle H. Nutrition need of mammalian cells in tissue culture. Science. 1955;122(3168):501-14.
5. van der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, et al. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol In Vitro. 2004;18(1):1-12.
6. Staines D, Price P. Managing serum requirements for cell culture. Baltimore:GIBCO® Cell Culture; 2003. p.87.
7. Johnson MC, Meyer AA, deSerres S, Herzog S, Peterson HD. Persistence of fetal bovine serum proteins in human keratinocytes. J Burn Care Rehabil. 1990;11(6):504-9.
8. Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11(2):228-32.
9. Vogt A, Mishell RI, Dutton RW. Stimulation of DNA synthesis in cultures of mouse spleen cell suspensions by bovine transferrin. Exp Cell Res. 1969;54(2):195-200.
10. Melchers F, Andersson J. Early changes in immunoglobin M synthesis after mitogenic stimulation of bone marrow derived lymphocytes. Biochemistry. 1974;13(22):4645-53.
11. Coutinho A, Möller G, Anderson J, Bullock WW. In vitro activation of mouse lymphocytes in serum-free medium: effect of T and B cell mitogens on proliferation and antibody synthesis. Eur J Immunol. 1973;3(5):299-306.
12. Irie RF, Irie K, Morton DL. Characteristics of heterologous membrane antigen on cultured human cells. J NatI Cancer Inst. 1974;53(6):1545-51.
13. Zielske JV, Golub SH. Fetal calf serum-induced blastogenic and cytotoxic responses of human lymphocytes. Cancer Res. 1976;36(10):3842-6.
14. Chachques JC, Herreros J, Trainini J, Juffe A, Rendal E, Prosper F, et al. Autologous human serum for cell culture avoids the implantation of cardioverter-defibrillators in cellular cardiomyoplasty. Int J Cardiol. 2004;95(Suppl 1):S29-33.
15. Johnson LF, deSerres S, Herzog SR, Peterson HD, Meyer AA. Antigenic cross-reactivity between media supplements for cultured keratinocyte grafts. J Burn Care Rehabil. 1991;12(4):306-12.
16. Eloit M. Risks of virus transmission associated with animal sera or substitutes and methods of control. Dev Biol Stand. 1999;99:9-16.
17. Falkner E, Appl H, Eder C, Losert UM, Schöffl H, Pfaller W. Serum free cell culture: the free access online database. Toxicol In Vitro. 2006;20(3):395-400.
18. Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010;27(1):53-62.
19. Chang RS. Continuous subcultivation of epithelial-like cells from normal human tissues. Proc Soc Exper Biol Med. 1954;87(2):440-3.
20. Ang LP, Tan DT, Seah CJ, Beuerman RW. The use of human serum in supporting the in vitro and in vivo proliferation of human conjunctival epithelial cells. Br J Ophthalmol. 2005;89(6):748-52.
21. Badrul AH, Aminuddin BS, Sharaf I, Samsudin OC, Munirah S, Ruszymah BH. The effects of autologous human serum on the growth of tissue engineered human articular cartilage. Med J Malaysia. 2004;59 Suppl B:11-2.
22. Yamamoto N, Isobe M, Negishi A, Yoshimasu H, Shimokawa H, Ohya K, et al. Effects of autologous serum on osteoblastic differentiation in human bone marrow cells. J Med Dent Sci. 2003;50(1):63-9.
23. Chuak KH, Aminuddin BS, Fuzinan NH, Ruszymah BH. Basic fibroblast growth factor with human serum supplementation: enhancement of human chondrocyte proliferation and promotion of cartilage regeneration. Singapore Med J. 2007;48(4):324-32.
24. Tallheden T, van der Lee J, Brantsing C, Månsson JE, Sjögren-Jansson E, Lindahl A. Human serum for culture of articular chondrocytes. Cell Transplant. 2005;14(7):469-79.
25. Kurita M, Aiba-Kojima E, Shigeura T, Matsumoto D, Suga H, Inoue K, et al. Differential effects of three preparations of human serum on expansion of various types of human cells. Plast Reconstr Surg. 2008;122(2):438-48.
26. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502-8.
27. Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117(Pt 5):667-75.
28. Cooper JT, Goldstein S. Comparative studies on human skin fibroblasts: life span and lipid metabolism in medium containing fetal bovine or human serum. In Vitro. 1977;13(8):473-6.
29. Mazlyzam AL, Aminuddin BS, Saim L, Ruszymah BH. Human serum is an advantageous supplement for human dermal fibroblast expansion: clinical implications for tissue engineering of skin. Arch Med Res. 2008;39(8):743-52.
30. Kondo H, Yonezawa Y, Ito H. Inhibitory effects of human serum on human fetal skin fibroblast migration: migration-inhibitory activity and substances in serum, and its age-related changes. In Vitro Cell Dev Biol Anim. 2000;36(4):256-61.
31. Kondo H, Nomaguchi TA, Yonezawa Y. Effects of serum from human subjects of different ages on migration in vitro of human fibroblasts. Mech Ageing Dev. 1989;47(1):25-37.
1. PhD, responsible for the Cell Culture and Wound Healing Research Laboratory (LIM 04) of the Plastic Surgery Department of Clinics Hospital of the Medical College of São Paulo University (HCFMUSP), São Paulo, SP, Brazil.
2. Resident Physician at the HCFMUSP, São Paulo, SP, Brazil.
3. Graduate student at the Medical College of São Paulo University, São Paulo, SP, Brazil.
4. M.Sc, Researcher of the Cell Culture and Wound Healing Research Laboratory from the Plastic Surgery Department of HCFMUSP, São Paulo, SP, Brazil.
5. PhD, responsible for the Tissue Bank of HCFMUSP Central Institute, São Paulo, SP, Brazil.
6. Plastic Surgery Resident Physician of HCFMUSP, São Paulo, SP, Brazil.
7. Postdoctoral Researcher at the Nuclear and Energetic Research Institute (IPEN), São Paulo, SP, Brazil.
8. Full Professor of Plastic Surgery Department of HCFMUSP, São Paulo, SP, Brazil.
Correspondence to:
César Isaac
Av. Doutor Arnaldo, 455 - sala 1360 - Pinheiros
São Paulo, SP, Brazil - CEP 01246-903
E-mail: cesaris@uol.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Received: March 23, 2011
Accepted: June 12, 2011
Study conducted at the Cell Culture and Wound Healing Research Laboratory (LIM 04) of the Plastic Surgery Department of Clinics Hospital of the Medical College of São Paulo University (HCFMUSP), São Paulo, SP, Brazil.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter