

Original Article - Year 2011 - Volume 26 -
Reconstruction of the abdominal wall in rats with hemicellulose
Hemicelulose em reconstrução da parede abdominal em ratos
ABSTRACT
Introduction: Loss of abdominal wall substance (LAWS) is clinically and surgically important because of its high incidence, the distress it causes, and the high cost of its current treatments. Recently, new techniques have been developed to treat LAWS, including the creation of flaps and use of biocompatible synthetic materials. The aim of this randomized uncontrolled experimental study was to assess the suitability of hemicellulose film for LAWS repair in rats. Methods: Forty female Wistar EPM III rats were divided into 4 groups (I-IV) of 10 animals each. All the animals received general anesthesia with 40 mg/kg intraperitoneal thiopental sodium. A 2.0 × 3.0 cm area of muscle-aponeurotic tissue and peritoneal damage was created in the anterior abdomen of every rat. Fifteen 2.0 × 3.0 cm hemicellulose films soaked in 0.9% saline were affixed in overlapping layers to the adjacent muscle walls with 8 separate 5-0 nylon sutures, and the skin and panniculus carnosus were synthesized by placing continuous 5-0 nylon sutures. The animals in groups I, II, III, and IV were euthanized after 3, 28, 35, and 240 days, respectively. Conclusion: Hemicellulose film is effective for repairing LAWS in rats.
Keywords: Abdominal wall/surgery. Implants, experimental. Rats. Histology.
RESUMO
Introdução: As perdas de substância da parede abdominal (PSPA) revestem-se de importância clínica e cirúrgica em função da alta frequência com que ocorrem, do sofrimento que produzem e do alto custo dos tratamentos atualmente ministrados. Nos últimos anos, tem-se observado o desenvolvimento de novas técnicas para a resolução das perdas de substância da PSPA, entre as quais se incluem a realização de retalhos e o uso de materiais biocompatíveis e sintéticos. Método: Esse estudo experimental aleatório e não controlado avaliou o uso da hemicelulose para a reparação das PSPA em 40 ratos Wistar EPM III, femêos. Os animais foram divididos em quatro grupos experimentais de estudo: I (n=10), II (n=10), III = (n=10) e IV (n=10). Todos os animais foram submetidos a anestesia geral utilizando-se o tiopental sódico, via intraperitonial. Foi provocada uma lesão de 2,0 x 3,0cm no tecido músculo-aponeurótico e peritônio da região anterior do abdome. A aposição da película de hemicelulose sobre a lesão foi realizada superpondo-se 15 películas de 2,0 x 3,0cm, umedecidas em soro fisiológico a 0,9%. A película foi fixada à parede muscular adjacente e, em seguida, foi realizada a síntese da pele e panículo carnoso por meio de sutura contínua com fio de náilon de número cinco zeros. A eutanásia dos animais dos grupos I (n=10), II (n=10), III (n=10) e IV (n=10) foi realizada após 3, 28, 35 e 240 dias, respectivamente. Conclusão: Esse estudo concluiu que a hemicelulose foi eficiente em reparar as grandes perdas da parede abdominal em ratos.
Palavras-chave: Parede abdominal/cirurgia. Implantes experimentais. Ratos. Histologia.
Loss of abdominal wall substance (LAWS) is clinically and surgically important because of its high incidence, the distress it causes, the bacterial growth in many cases, and the high cost of the current treatments.
A challenge for surgeons today is the ability to close the abdominal wall when the margins of the muscle-aponeurotic flap are widely separated1. The extensive tissue loss after trauma or tumors, swollen extrusion of the viscera through the site of injury, and weakening of the abdominal wall by infection are some factors limiting the success of primary closure of LAWS. Consequently, wound dehiscence, incisional hernia, and evisceration may occur. To minimize the risk of such complications, many surgeons do not perform primary closure of the lesion, some await full homeostatic stabilization of the patient, and others prefer primary closure by suturing to avoid sequelae such as heat and fluid loss, hypermetabolism, and occurrence of enteral fistula2.
Recently, new techniques have been developed for LAWS repair, including the creation of flaps and use of biocompatible synthetic materials3-6. At present, prostheses derived from biocompatible synthetic materials are frequently used to reconstruct the abdominal wall, mainly because of the simplicity of the surgical procedure and reduced need for additional surgeries, making such reconstruction the procedure of choice in many countries6,7.
Materials for treating LAWS have been the subject of research since the late nineteenth century, when Witzel used a silver prosthesis for reconstructing the abdominal wall8. Burke9 introduced tantalum, but this material was abandoned after evidence of its tendency for fragmentation and frequent complications such as small intestinal fistula formation, ulceration, and extrusion of metal fragments through the peritoneum and skin10. Since the 1950s, a variety of synthetic polymers have been available: polyethylene terephthalate (Dacron®)11; monofilament polypropylene (Marlex®) and double-stranded polypropylene (Prolene®)12; nonporous multifilament polytetrafluoroethylene (Teflon®), which was abandoned soon after; and expanded polytetrafluoroethylene (ePTFE), which is porous and causes less foreign body reaction13. Later, PTFE was developed into a form marketed as Gore-Tex®8, which is also used for reconstructing the abdominal wall.
The international literature describes many other materials for LAWS repair: multifilament polyester (Mersilene®)14; compounds of lactic, glycolic, and polyglycolic acids, polyglactin, and their variants (Vicryl®)13,15, derived from collagen16; monofilament polyamide (nylon)17; porcine small intestinal submucosa18; bovine pericardium treated with glutaraldehyde19; combination of polypropylene and polyglactin 910 (SHM)6; total skin autologous graft20; dehydrated human dura mater (Tutoplast®)20,21; combination of polypropylene and ePTFE (Bard Composix)22; polyvinylidene fluoride (PVDF)23; polyurethane coated with polyethylene (polyester composite)24; and combination of polypropylene and polyglactin multifilament (Vypro II)7.
Despite the large number of available synthetic biocompatible materials, the various materials used in medicine have more specific disadvantages than advantages23, such as intestinal adhesion to the prosthesis, some degree of inflammatory response and fibroplasia, resorption of the material, encapsulation, and tumor development after implantation of the material. These common factors influence the choice of prosthesis and encourage research on new materials.
Hemicellulose film, developed through a biotechnological process, was registered and patented in 1990 (DIMED/MS 8306340 Brazil) by Lauro Xavier Filho and Paulo Marçal de Queiroz, and its application was recognized soon thereafter by Solas et al.25 It is currently used as a temporary replacement of human skin in superficial lesions after burns and bruises26. The aim of this study was to assess the suitability of hemicellulose film for LAWS repair in rats.
METHODS
This randomized uncontrolled experimental study was performed at the Animal Center of the Universidade Federal de Alagoas (UFAL).
The hemicellulose film under study was obtained from cultures of Acetobacter xylinum, Saccharomyces cerevisiae, and Saccharomyces pombe. It is composed of glucosamine, N-acetyl-galactosamine, amino acids, and carbohydrates; has no additives or chemical modifications; is cream colored, with a tendency toward white; has an average thickness of 0.05 mm; is resistant to tensile strength; has an average degree of flexibility; is heat resistant up to 150ºC; and has an uneven surface allowing limited gas exchange (Figure 1).

Figure 1 - Hemicellulose film.
Forty female Wistar EPM III rats aged 4-6 months and weighing 200-300 gm were used. The animals were kept indoors, properly cooled at 22ºC under artificial light, and received water and food ad libitum. They received daily care, their cages were changed twice weekly, and they were observed for at least 15 days before the experiments, as recommended by Federal Law 6638.
The animals were randomly divided into 4 distinct groups (I-IV) of 10 rats each, as shown in Table 1. The following experimental procedures were then performed: anesthesia, surgery, and euthanasia.

Anesthesia
Before the experimental procedures, the animals were properly weighed on an electronic scale (Filizola MF3). All animals received general anesthesia with 40 mg/kg intraperitoneal thiopental sodium (1 gm powder diluted in 40 ml of 0.9% sterile saline). Induction was achieved in about 10 min, and anesthesia lasted for around an hour. For prolonged surgery, quarter of the initial anesthetic dose was injected. After anesthesia, the animal was fixed in the supine position on a 20 x 30 cm cork board by using adhesive tape. The abdominal hair was manually epilated, and antisepsis was ensured using povidone-iodine detergent.
Surgery
The surgical procedure was initiated by a longitudinal midline incision from the xiphoid process to the lower portion of the rectus abdominis of the animal. The skin and panniculus carnosus were separated from the abdominal wall muscles, and 1 cm below the xiphoid process, an injury site previously demarcated with a 2.0 x 3.0 cm template (2 cm wide laterolaterally and 3 cm long craniocaudally) was created by removing a similar-sized fragment of muscle-aponeurotic tissue and peritoneum of the anterior abdomen (Figure 2). Fifteen 2.0 x 3.0 cm hemicellulose films soaked in 0.9% saline were affixed in overlapping layers to the site of injury (Figure 3). The films were fixed to the adjacent muscle walls with 8 separate 5-0 nylon sutures (Figure 4). Subsequently, the skin and panniculus carnosus were synthesized by continuous suturing with 5-0 nylon thread.

Figure 2 - Experimental tissue damage (2.0 x 3.0 cm) in the anterior abdomen.

Figure 3 - Specimen of the muscle-aponeurotic tissue and peritoneum of the anterior abdomen and affixation of hemicellulose film measuring 2.0 x 3.0 cm.

Figure 4 - Hemicellulose film (moistened with saline) placed over the lesion and affixed to the adjacent muscle wall.
All the animals were examined daily after the surgery for infection, abdominal herniation, dehiscence, serous secretion, and bruises.
Euthanasia
Before euthanasia, containers with lids containing 50 ml of 10% formalin were prepared for preserving tissue specimens of the anterior abdomen. These containers were properly identified with labels including the following data: species, identification code for the animals (marking of the animal ear), date of birth, date of surgery, type of surgery with a description of the surgery, weight on the day of surgery, date of euthanasia, weight after euthanasia, and identification of the blade used for the anatomopathological examination.
Each animal was euthanized with 80 ml of intraperitoneal sodium thiopental, and after death, the body was weighed by using a digital scale. Subsequently, the abdominal fragment containing the area of study was removed while preserving the tissue integrity. Each fragment was immersed in saline solution for cleaning and soon thereafter placed, with the film side up, in the appropriately labeled container with formalin.
The animals in groups I, II, III, and IV were euthanized after 3, 28, 35, and 240 days, respectively. Thereafter, all the animals were again observed for signs of wound infection, herniation, dehiscence, serous secretion, bruises on the anterior abdomen, and film adhesion to the viscera. Subsequently, the abdominal fragments were preserved in 10% formalin and sent to the Department of Pathology, University Hospital, UFAL, for analysis.
The macroscopic and microscopic analyses of the abdominal fragments containing the films were conducted by a single researcher and pathologist of the University Hospital (UFAL). All results of the anatomopathological examinations were evaluated by the same team members, who filled individual questionnaires with all the macroscopic and microscopic data of the animals (Table 2).

Tissue sections were obtained from paraffin-embedded tissue blocks. In this process, tissue blocks were dehydrated in ethanol and 3- to 4-mm-thick sections were obtained by using a microtome (Jung/Reisterstown). Tissue staining was performed with hematoxylin and eosin for epithelial cells, Gomori's trichrome for collagen and connective tissue, Verhoeff stain for elastic fibers, and silver impregnation for reticular fibers (reticulin).
The results were evaluated on the basis of the macroscopic and microscopic analyses of inflammatory reactions in response to the film, presence of granulation tissue, film resorption, neovascularization, fibroplasia, film adherence to the viscera, encapsulation, and tumor development.
Statistical Methods
Epi-Info 2002, software developed by the Centers for Disease Control and Prevention (Atlanta, GA, USA), was used for the database and statistical analysis at the level of 95%.
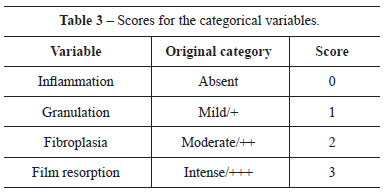
Scores were applied to the results of the categorical variables (Table 3). Hypothesis H0 (equal), from the scores of each variable in the groups, was tested by ANOVA when Bartlett's test indicated homoscedasticity; otherwise, the Kruskal-Wallis test was applied at the 95% confidence interval. The linear regression with scatter plot was generated to evaluate correlations between the variables.
RESULTS
The animals in groups I and II showed no infection, abdominal herniation, dehiscence, hematoma, and serous secretion. In group III, one rat developed dehiscence and two animals had serous secretion. In group IV, one case each of dehiscence and serous secretion were noted (Table 4).
The results of the microscopic evaluations are presented in (Table 5 and Figures 5 to 15).

Figure 5 - Group-wise distribution (%) of inflammation.

Figure 6 - Group-wise distribution (%) of granulation.

Figure 7 - Group-wise distribution (%) of fibroplasia.

Figure 8 - Group-wise distribution (%) of film resorption.

Figure 9 - Group-wise distribution (%) of film adherence to the viscera.

Figure 10 - Group-wise distribution (%) of encapsulation.

Figure 11 - Scatter and regression plots comparing the degree of fibroplasia and extent of film resorption.

Figure 12 - Scatter and regression plots comparing the degrees of inflammation and fibroplasia.

Figure 13 - Scatter and regression plots comparing the degrees of granulation and inflammation.

Figure 14 - Scatter and regression plots comparing the degree of granulation and extent of film resorption.

Figure 15 - Scatter and regression plots comparing the extent of film resorption and degree of inflammation.
Film adhesion to the viscera, encapsulation, and tumor development were not scored.
Figures 5 to 10 demonstrate that inflammation decreased over time, granulation was more prevalent in groups II and III, fibroplasia increased over time, film resorption was complete in group IV, intestinal adhesion was absent in groups I and III and hardly present in groups II and IV, and encapsulation was clearly present in groups II-IV.
ANOVA confirmed that the differences in the parameters between the groups were significant: inflammation, granulation, fibroplasia, and film resorption were more intense in groups I, II, IV, and IV, respectively.
DISCUSSION
LAWS is still a major public health problem and the ideal material to repair the abdominal wall remains a challenge in modern surgery. The characteristics of the ideal material were postulated by Scales in 1953 and later described by Roa27. According to Scales, the ideal replacement of human tissue should not cause inflammatory reaction, foreign body reaction, hypersensitivity reaction, and carcinogenesis; should be chemically inert and be able to endure stresses and tensile strength; should be sterilizable; should not be modified by tissue conditions; and should be manufacturable in series27.
The objective of this experimental study was to assess the ability of hemicellulose film to repair part of the abdominal wall of rats. However, the tensile strength of the film and the stiffness and motility of the intestines were not studied; further, no device was used to induce abdominal infection. The study focused on individual assessments of the microscopic and macroscopic aspects of hemicellulose film affixed to the damaged area of the abdominal wall (experimentally induced LAWS) of female Wistar EPM III rats (n=40).
Hemicellulose film (Bioskin®) consists of glucosamine, N-acetyl-galactosamine, amino acids, carbohydrates, and polysaccharides obtained by culture of Acetobacter xylinum, Saccharomyces cerevisiae, and the Saccharomyces pombe. Cellulose is an unbranched polymer of glucose residues arranged in a linear chain, where each glucose residue is rotated approximately 180º. Glucosamine is found in high concentrations in the joint space and stimulates the production of substances for treating inflammatory joint lesions28. N-acetyl galactosamine is formed by galactose containing an amino group together with acetyl obtained by acetylation28. Acetobacter xylinum is a gram-negative bacterium involved in the synthesis of cellulose and the model organism for studying enzymes and genes involved in the biosynthesis of cellulose29. Saccharomyces cerevisiae and Saccharomyces pombe are fungi that have nuclei scattered in mycelia, do not have plastids or photosynthetic pigments, and absorb their nutrition from the culture medium29.
The film used in this study resembles BioFill®, a cellulose film produced by biosynthesis from bacteria of the genus Acetobacter and studied as a biological dressing for treating burns and abrasions due to trauma or surgery such as dermabrasion30-32. Cellulose film has been used as a temporary substitute of human skin. Clinically®, BioFill undergoes dehydration and develops a thick brownish crust when placed over damaged tissue of a bruise or burn. This material is considered to provide a satisfactory and effective temporary covering for shallow injuries and small areas of mobility. Its adhesion to the wound surface decreases pain and blood and electrolyte loss, and its transparency enables constant inspection of the wound and prompt treatment if complications occur. Silva et al.33, Tucci et al.34, and Legaz et al.26 used hemicellulose film and reported findings similar to those of the studies on BioFill.
The reason for using hemicellulose film (Bioskin®) as the replacement for the damaged abdominal wall of rats was to find a low-cost biological material capable of integration with minimal tissue reactions and minimal adhesion to the intestines in the immediate or long-term postoperative period, without malignant transformation (e.g., metaplasia, dysplasia, and malignant tumor), and without requiring its removal in cases of local infection.
Although the use of rats as experimental animals does not exactly reproduce the situation in humans, Wistar rats have advantages such as availability, ease of handling, low cost, and lack of many ethical and bioethical considerations applied to humans35-37. All the experimental animals were operated between 4 and 6 months of age while weighing 200-300 gm to minimize the effects of age and weight on the process of healing and avoid the influence of a negative metabolic response in the animal35.
Intraperitoneal sodium pentobarbital was chosen as the anesthetic to minimize the respiratory insufficiency caused by ethyl ether, because it is as safe as ketamine hydrochloride20,38,39, and because it has been extensively used in similar studies40-42.
In all the animals, the average lesion size was 2.0 x 3.0 cm, including the muscle-aponeurotic tissue of the abdominal wall. The dimensions of the lesion followed the precepts of Milton43 on scarring and are very close to the dimensions proposed by Walter et al.16, Birbilis et al.44, and Hooker et al.45 (3.0 x 4.0 cm) and by Baptista et al.46 (2.5 x 2.5 cm).
The weight of the animals, measured individually at the beginning and end of the experiment, showed the expected normal changes. The animals in group I (n=10), euthanized 3 days after the surgery, lost weight; those in group II (n=10), sacrificed after 4 weeks, presented no significant weight loss; and the animals in groups III (n=10) and IV (n=10) gained considerable weight. These findings are consistent with the good health of the animals in the postoperative period.
In all the animals, the films were sutured to the muscle-aponeurotic tissue with 5-0 nonabsorbable monofilament nylon, which is considered the most appropriate material47. However, Toosie et al.39 demonstrated that topical use of fibrin glue to replace sutures reduces intestinal adherence after the reconstruction of hernias in rats with prostheses. Fibrin glue was not used in the present study.
The ideal histological method would be the one causing a minimal shift from the in vivo condition (found immediately before euthanasia) and allowing maximal detection of all tissue parameters. The requirement for good pathological examination is the use of a fixative solution causing the lowest amount of precipitation, and therefore, all the tissue specimens were prepared with formaldehyde, allowing the best results for optical microscopy.
None of the experimental animals presented herniation of the abdominal wall after the reconstructive surgery. The macroscopic analysis of the tissue segments containing the films revealed visceral adhesions in rat 9 of group II and rats 7 and 9 of group IV, which confirm the results of the anatomopathological examination and are consistent with the reports of Cnota et al.2 and Jenkins et al.15 Microscopic analysis revealed that the surgically inserted hemicellulose films were well accepted by the rats and were capable of preventing abdominal herniation and maintaining equilibrium with the surrounding tissues. These findings confirm that hemicellulose film is a well-tolerated implant material, as previously described by Roa27.
Statistical tests were applied to evaluate the scarring outcome, without comparing hemicellulose film with other materials used for LAWS repair. The linear regression and scatter plots (Figures 11-15) showed weak correlations for analyses involving granulation. This finding is comprehensive because granulation had the highest score in Group II, without increasing or decreasing consistently over time. The correlations of the other variables were very good, except the correlation between film resorption and inflammation, which was good. The downward trend of the curves between inflammation and fibroplasia, and between film resorption and inflammation is an expected phenomenon. The observed regression results are consistent with the expected features of a healing process within the limits of normality.
The obtained significant differences confirm that the healing process after the implantation of hemicellulose film was satisfactory and evolved without causing abnormalities in the study groups (I-IV).
CONCLUSION
Hemicellulose film is effective for repairing LAWS in rats. None of the animals presented herniation of the abdominal wall. The macroscopic analysis revealed adhesions in rat 9 of group II and rats 7 and 9 of group IV, which were confirmed by the anatomopathological examinations. Microscopic analysis revealed that hemicellulose film was effective to restrain the intestinal viscera from herniation.
REFERENCES
1. Kapan S, Kapan M, Goksoy E, Karabicak I, Oktar H. Comparison of PTFE, pericardium bovine and fascia lata for repair of incisional hernia in rat model, experimental study. Hernia. 2003;7(1):39-43.
2. Cnota MA, Aliabadi-Wahle S, Choe EU, Jacob JT, Flint LM, Ferrara JJ. Development of a novel synthetic material to close abdominal wall defects. Am Surg. 1998;64(5):415-8.
3. Chung S, Hazen A, Levine JP, Baux G, Olivier WA, Yee HT, et al. Vascularized acellular dermal matrix island flaps for the repair of abdominal muscle defects. Plast Reconstr Surg. 2003;111(1):225-32.
4. de Vries Reilingh TS, van Goor H, Rosman C, Bemelmans MH, de Jong D, van Nieuwenhoven EJ, et al. "Components separation technique" for the repair of large abdominal wall hernias. J Am Coll Surg. 2003;196(1):32-7.
5. Dumanian GA, Denham W. Comparison of repair techniques for major incisional hernias. Am J Surg. 2003;185(1):61-5.
6. Klinge U, Klosterhalfen B, Conze J, Limberg W, Obolenski B, Ottinger AP, et al. Modified mesh for hernia repair that is adapted to the physiology of the abdominal wall. Eur J Surg. 1998;164(12):951-60.
7. Junge K, Klinge U, Rosch R, Klosterhalfen B, Schumpelick V. Functional and morphologic properties of a modified mesh for inguinal hernia repair. World J Surg. 2002;26(12):1472-80.
8. Bauer JJ, Salky BA, Gelernt IM, Kreel I. Repair of large abdominal wall defects with expanded polytetrafluoroethylene (PTFE). Ann Surg. 1987;206(6):765-9.
9. Burke GL. The corrosion of metals in the tissues; and an introduction to tantalum. Can Med Assoc J. 1940;43(2):125-8.
10. Adler RH. An evaluation of surgical mesh in the repair of hernias and tissue defects. Arch Surg. 1962;85:836-44.
11. Wolstenholme JT. Use of commercial dacron fabric in the repair of inguinal hernias and abdominal wall defects. AMA Arch Surg. 1956;73(6):1004-8.
12. Usher FC. A New technique for reparing large abdominal wall defects. Arch Surg. 1961;82:870-7.
13. Lamb JP, Vitale T, Kaminski DL. Comparative evaluation of synthetic meshes used for abdominal wall replacement. Surgery 1983;93(5):643-8.
14. Cerise EJ, Busuttil RW, Craighead CC, Ogden WW 2nd. The use of Mersilene mesh is repair of abdominal wall hernias: a clinical and experimental study. Ann Surg. 1975;181(5):728-34.
15. Jenkins SD, Klamer TW, Parteka JJ, Condon RE. A comparison of prosthetic materials used to repair abdominal wall defects. Surgery. 1983;94(2):392-8.
16. Walter M, Brenner U, Holzmuller W, Muller JM. Experiments with a biological material for the closure of incisional hernias. Lab Anim. 1987;21(3):195-200.
17. Nyhus LM, Condom RE. Hernia. 3ª ed. Philadelphia:Lippincott;1989.
18. Prevel CD, Eppley BL, Summerlin DJ, Jackson JR, McCarty M, Badylak SF. Small intestinal submucosa: utilization for repair of rodent abdominal wall defects. Ann Plast Surg. 1995;35(4):374-80.
19. Olmos Zúñiga JR, Jasso Victoria R, Sotres Vega A, Arreola Ramírez JL, Gaxiola Gaxiola M, Vanda Cantón B, et al. Utilización de bioprótesis liofilizadas en la reparacíon de defectos abdominales en ratas. Rev Inst Nal Enf Resp Méx. 1996;9(3):183-6.
20. Kama NA, Coskun T, Yavuz H, Doganay M, Reis E, Akat AZ. Autologous skin graft, human dura mater and polypropylene mesh for the repair of ventral abdominal hernias: an experimental study. Eur J Surg. 1999;165(11):1080-5.
21. Baykal A, Yorganci K, Sokmensuer C, Hamaloglu E, Renda N, Sayek I. An experimental study of the adhesive potential of different meshes. Eur J Surg. 2000;166(6):490-4.
22. Ferrando JM, Vidal J, Armengol M, Gil J, Manero JM, Huguet P, et al. Experimental evaluation of a new layered prosthesis exhibiting a low tensile modulus of elasticity: long-term integration response within the rat abdominal wall. World J Surg. 2002;26(4):409-15.
23. Klinge U, Klosterhalfen B, Ottinger AP, Junge K, Schumpelick V. PVDF as a new polymer for the construction of surgical meshes. Biomaterials. 2002;23(16):3487-93.
24. Zieren J, Paul M, Osei-Agyemang T, Maecker F, Muller JM. Polyurethane-covered dacron mesh versus polytetrafluoroethylene DualMesh for intraperitoneal hernia repair in rats. Surg Today 2002;32(10):884-6.
25. Solas MT, Vicente C, Xavier L, Legaz ME. Ionic adsorption of catalase on bioskin: kinetic and ultrastructural studies. J Biotechnol. 1994;33(1):63-70.
26. Legaz ME et al. Quaternary structure of catalase using a bioskin-immobilized enzyme. Biotechnology Techniques. 1998;12(6):481-4.
27. Roa TT. Materiales inertes. In: Coiffman F, ed. Texto de cirurgia plastica, reconstructiva y estetica. Barcelona: Salvat;1986. p.182-5.
28. Baici A, Hörler D, Moser B, Hofer HO, Fehr K, Wagenhäuser FJ. Analisis of glycosaminoglycans in human serum after oral administration of chondroitin sulfate. Rheumatol Int. 1992;12(3):81-8.
29. Fontana JD, Franco VC, de Souza SJ, Lyra IN, de Souza AM. Nature of plant stimulators in the production of Acetobacter xylinum ("tea fungus") biofilm used in skin therapy. Appl Biochem Biotechnol. 1991;28-29:341-51.
30. Cabral LM. Curativo biológico no tratamento do grande queimado: apresentação de caso. Rev Bras Cir. 1987;77(6):383-9.
31. Pitanguy I, Salgado F, Maracajá PF. Utilização de película de celulose (Biofillâ) como curativo biológico. Rev Bras Cir. 1988;78(5):317-26.
32. Rebello C, Almeida DA, Lima Júnior EM, Dornelas MP. Bio-fill: um novo substituto de pele: nossa experiência. Rev Bras Cir. 1987;77(6):407-14.
33. Silva SC, Sperança PA, Gusmão ES. Avaliação clínica da membrana biológica Bioskin ®(Hemicelulose) na técnica de regeneração tecidual guiada. Periodontia. 1995;5(2):233-42.
34. Tucci MG et al. Valutazione dell'efficacia clinica e della tollerabilità di un nuovo biomateriale per il trattamento delle perdite di sostanza della cute. Chron. Derm. 1996;6(4):499-507.
35. Donovan WE. Modelos experimentales en la investigación de los colgajos cutáneos. In: Grabb WC, Myers MB, eds. Colgajos cutáneos. Barcelona: Salvat, 1982. p. 11-20. Versão espanhola.
36. Lawrence WT, Murphy RC, Robson MC, Heggers JP. The detrimental effect of cigarette smoking on flap survival: an experimental study in the rat. Br J Plast Surg. 1984;37(2):216-9.
37. van Adrichem LN, Hoegen R, Hovius SE, Kort WJ, van Strik R, Vuzevski VD. The effect of cigarette smoking on the survival of free vascularized and pedicled epigastric flaps in the rat. Plast Reconstr Surg. 1996;97(1):86-96.
38. Raman J, Montano SR, Lord RS. Assisted local anaesthesia in rabbits. Microsurgery. 1989;10(1):75-6.
39. Toosie K, Gallego K, Stabile BE, Schaber B, French S, de Virgilio C. Fibrin glue reduces intra-abdominal adhesions to synthetic mesh in a rat ventral hernia model. Am Surg. 2000;66(1):41-5.
40. Klinge U, Klosterhalfen B, Muller M, Schumpelick V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg. 1999;165(7):665-73.
41. Klosterhalfen B, Klinge U, Schumpelick V. Functional and morphological evaluation of different polypropylene-mesh modifications for abdominal wall repair. Biomaterials. 1998;19(24):2235-46.
42. Simmermacher RK, Schakenraad JM, Bleichrodt RP. Reherniation after repair of the abdominal wall with expanded polytetrafluoroethylene. J Am Coll Surg. 1994;178(6):613-6.
43. Milton SH. The tubed pedicle flap. Br J Plast Surg. 1969;22(1):53-9.
44. Birbilis T, Theodoropoulou E, Birbili A, Dimas S, Leutsakos V. A preliminary report on the use of relon mesh in the repair of eventrations with large parietal defects. An experimental study in rats. J Int Med Res. 1997;25(3):135-40.
45. Hooker GD, Taylor BM, Driman DK. Prevention of adhesion formation with use of sodium hyaluronate-based bioresorbable membrane in a rat model of ventral hernia repair with polypropylene mesh: a randomized, controlled study. Surgery. 1999;125(2):211-6.
46. Baptista ML, Bonsack ME, Delaney JP. Seprafilm reduces adhesions to polypropylene mesh. Surgery. 2000;128(1):86-92.
47. Bucknall TE. Factors influencing wound complications: a clinical and experimental study. Ann R Coll Surg Engl. 1983;65(2):71-7.
1. Head of the Plastic and Reconstructive Surgery Service of the University Hospital, Federal University of Alagoas (UFAL) and Coordinator of the Discipline of Plastic Surgery, Faculty of Medicine at UFAL, Maceió, AL, Brazil.
2. Surgeon of Head and Neck at the Reconstructive and Plastic Surgery Service, University Hospital of UFAL, Division of Tumors, Maceió, AL, Brazil.
3. Physician graduate at UFAL, Discipline of Plastic Surgery, Faculty of Medicine at UFAL, Maceió, AL, Brazil.
4. Medical Student from the Faculty of Medicine at UFAL, Maceió, AL, Brazil.
5. Head of the Discipline and the Plastic Surgery Service from Federal University of São Paulo, São Paulo, Brazil.
Corresponding author:
Fernando Gomes de Andrade
Rua José Freire Moura, 191/104 - Ponta Verde
Maceió, AL, Brazil - CEP 57000-000
E-mail: fernandogomes1911@hotmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Received: January 27, 2011
Accepted: March 10, 2011
Work performed at the Universidade Federal de Alagoas - (Plastic and Reconstructive Surgery Service of the University Hospital - UFAL), Maceió, AL, Brazil.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter