

Original Article - Year 2011 - Volume 26 -
Silicone gel breast implant insertion by the axillary route using the partial submuscular location without videoendoscopic assistance
Implante mamário de silicone gel em posição submuscular parcial, via axilar, sem o emprego de videoendoscopia
ABSTRACT
Introduction: Submuscular and subglandular locations for silicone gel breast implant insertion have their advantages and disadvantages; a combined implant-insertion plane could yield the best results. Objective: The aim of this study was to assess the outcome of breast augmentation with silicone gel implants by the axillary route using the partial submuscular location without videoendoscopic assistance. Methods: The medical records of 27 patients who underwent silicone gel breast implant insertion by the axillary route using the partial submuscular location without videoendoscopic assistance between 2006 and 2008 were retrospectively reviewed. The surgical results were assessed by subjective questionnaires. Results: Twenty patients had axillary retraction, two developed seroma, and one experienced capsular contracture. The procedure was considered painful, mainly in the breasts, by 29% of the patients. Only 1 patient did not classify her scar as good or excellent, but all considered their results as natural and believed that the muscle flap helped to achieve this goal.
Keywords: Breast Implantation. Pectoral Muscles. Breast/surgery.
RESUMO
Introdução: Os planos submuscular e subglandular para acomodar implantes mamários têm suas vantagens e desvantagens. Para tentar obter o melhor de cada uma destas alternativas, propõem-se os planos mistos. Objetivo: Avaliar a via axilar para a realização do plano misto na inserção do implante de silicone mamário sem videoendoscópio. Método: Estudo retrospectivo dos 27 pacientes operados pelo autor com esta técnica, no período de 2006 a 2008, foi realizado. Foram avaliados os resultados por questionário subjetivo, respondido pelas pacientes e pela revisão de seus prontuários. Resultados: As complicações e suas respectivas ocorrências foram: retrações axilares (20 casos); seromas (2 casos); contratura capsular (1 caso). As pacientes consideraram o procedimento como doloroso em apenas 29% dos casos, principalmente nas mamas. Apenas uma paciente não classificou a sua cicatriz como boa ou excelente. Todas acharam seu resultado natural e acreditam que o retalho muscular ajudou neste objetivo.
Palavras-chave: Implante Mamário. Músculos Peitorais. Mama/cirurgia.
Silicone gel breast implantation is one of the most commonly performed esthetic surgeries. The access routes to insert such implants vary widely. Among them, the axillary route is one of the most controversial, mainly because of the greater learning curve, the presence of important neural and vascular structures in the axilla1, the possibility of altering the breast lymphatic drainage, and the difficulty in properly viewing the insertion planes without videoendoscopic aid. However, this technique produces a scar in a region that is barely visible.
Mixed implant-insertion planes involve the positioning of silicone gel breast implants partially under muscle and glandular tissue in several ways. The partial submuscular location involves implant insertion under the upper portion of the pectoralis major by dilatation of the muscle and minimal release of its medial attachment in the ribs and sternum. It ensures the most natural outcome of the prosthesis in the upper medial third, avoiding some specific complications of the submuscular location2.
The aim of this study was to assess the outcome of breast augmentation with silicone gel implants by the axillary route using the partial submuscular location without videoendoscopic assistance by reviewing outpatient medical records and questionnaires filled by patients.
METHODS
The medical records of 27 patients (mean age, 24.3 years; age range, 18-36 years) who underwent silicone gel breast implant insertion by the axillary route using the partial submuscular location without videoendoscopic assistance between 2006 and 2008 were retrospectively reviewed. The institutional ethics committee approved the study.
Each patient herself chose the access route (sulcus, axillary, or periareolar) after receiving explanations regarding their benefits and complications. The partial submuscular location was suggested for patients with poor breast coverage in the upper third. The preoperative preparation included mammography, electrocardiography, blood tests, and a preanesthetic visit. The patients were asked to suspend the use of oral contraceptives 15 days before the procedure and 10 days postoperatively. The use of diet pills and natural medications was suspended at least 10 days before the surgery.
All the patients were operated on by using the following technique:
The properly anesthetized patient was placed in the supine position with the arms abducted at 90º from the body. After ensuring antisepsis and asepsis with sterile drapes, 100 ml saline with 1:400,000 epinephrine was injected in each breast. A 4-cm-long straight incision was made parallel and 2 mm inferior to the axillary fold. The safety point at the anterior edge of the incision was marked with 3.0 nylon sutures. The skin from the armpit to the edge of the pectoralis major was detached superficially, with the aid of the light source, to avoid entering the axillary fat. Prefascial undermining with a simple breast dissector was performed in the upper-to-lower and medial-to-lateral directions (Figure 1).
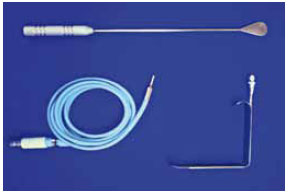
Figure 1 - Breast dissector and optical fiber.
The pectoralis major fascia was incised with scissors to view the muscle and the breast dissector was used to create a submuscular pouch in the upper third of the axillary space. Strict hemostasis of the armpit was maintained, but no drains were used. A silicone gel breast implant was then inserted through the created tunnel and accommodated in the mixed space (Figure 2).
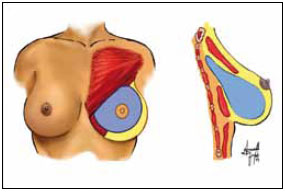
Figure 2 - Muscle detachment.
Baroudi points in the axillary hollow were sutured with 4.0 nylon and Monocryl 4.0 skin sutures were placed in two planes. Finally, a gauze and micropore dressing were placed, followed by a surgical bra.
The patients answered a subjective questionnaire (Table 1) between 6 and 9 months after the surgery. This questionnaire sought to assess the technique in terms of naturalness of the result, patient satisfaction, feeling upon palpation, time of return to daily activities, and level of postoperative pain. The medical records and photographs were reviewed and the following parameters were analyzed: operative time, length of hospitalization, early and late complications, reoperation, type of anesthesia, and results.
RESULTS
The average operative time was 74 min, ranging from 50 to 120 min. All patients received epidural anesthesia. The implant volume ranged from 260 to 385 ml. The length of hospitalization was less than 12 h in all cases. Only two patients underwent associated procedures (rhinoplasty and protrusion of the papilla). One patient underwent postoperative radiotherapy for hypertrophic scar prophylaxis because of a history of poor healing.
Only two patients had surgical complications: one experienced burning in the right forearm due to malfunctioning of the electric cautery and the other had difficult hemostasis, increasing the intraoperative time. No bruises, axillary lacerations, and difficulty in achieving breast symmetry or space detachment for prosthesis insertion were encountered. Further, no cases of late dislocation of the prosthesis were observed.
During the outpatient follow-up, two (7%) cases of seroma were noted between the tenth and the fifteenth day after surgery that were resolved with a single puncture of 20 and 30 ml, respectively. One patient had wine-colored streaks in one breast, which resolved with medical treatment. Twenty (74%) patients had axillary retraction; in 18 patients, the retraction disappeared spontaneously within 3 months, whereas 1 patient required more than 6 months for spontaneous disappearance and 1 patient required surgical release (Figure 3).
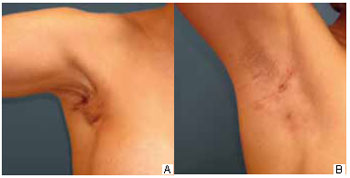
Figure 3 - A: Intense axillary retraction over a two-month postoperative period. B: Same patient who received conservative treatment and was examined 6 months postoperatively
Four patients had paresthesia or pain in the inner arm, probably due to injury or traction of the intercostobrachial nerve, which spontaneously resolved within 45 days. This complication occurred in the first case because of deeper dissection of the axillary fat, which must remain intact1. Even with this nerve injury, recovery is observed once several branches of this nerve merge with the medial brachial cutaneous nerve3. Another hypothesis for the paresthesia is intercostobrachial nerve traction by fibrotic ridges, a fact that reinforces the simultaneous improvement of both complications3. Capsular contracture occurred only in one breast (Grade II), but capsulectomy was not necessary.
The following results were obtained by the subjective questionnaires:
Access route: Seventeen (62%) patients chose the axillary route because they did not want scars on the breasts, 6 (22%) did so at the surgeon's suggestion, and 4 (14%) believed that the scar would be more hidden in the armpit by this route. Time of return to daily activities: Fifteen (55%), 5 (18%), and 2 (7%) patients started their routine activities in the second, third, and fourth weeks, respectively. All of them were able to raise their arms up to the shoulder with mild pain. The remaining 5 patients were able to drive without any problem within the first 15 days. Level of postoperative pain: Eight (29%), 12 (44%), and 7 (30%) patients considered the surgery extremely painful, moderately painful, and mildly painful, respectively. However, only 5 (18%) patients reported the greatest pain in the armpit; the others experienced the most intense pain in the breast. Axillary retraction: Because axillary retraction was the most common complication in the postoperative period, the patients were asked about the feeling these adhesions caused. Fourteen (51%) patients reported some discomfort with the retractions, 6 (22%) patients were very uncomfortable, and 7 (25%) patients had no discomfort. Scar assessment: Fifteen (55%) patients considered the scars as excellent, 11 (41%) thought them to be good, and 1 (4%) reported a bad scar (Figure 4). Coincidentally, the patients who rated the scars as excellent had a longer evolution time (> 9 months after the surgery).
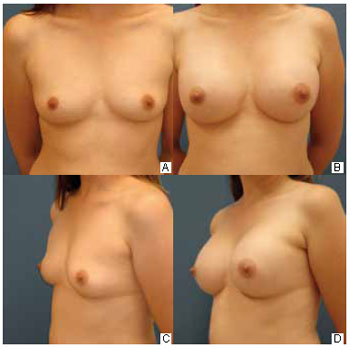
Figure 4 - A: Preoperative anterior view. B: Six-month postoperative previous view. C: Preoperative three-quarter view. D: Six-month postoperative three-quarter view.
Naturalness and efficiency of the partial submuscular location to hide the prosthesis: Twenty (74%) patients were concerned about the naturalness of the result before the procedure, but all (100%) were satisfied postoperatively. All patients (100%) believed that the muscle flap helped to achieve this result (Figure 5).
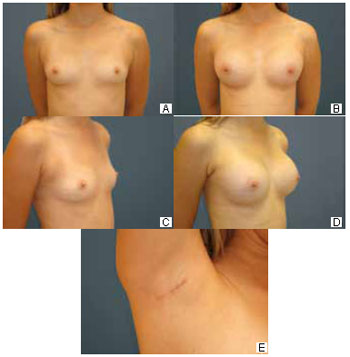
Figure 5 - A: Preoperative anterior view. B: Six-month postoperative previous view. C: Preoperative three-quarter view. D: Six-month postoperative three-quarter view. E: Axillary hollow at 6 months postoperatively.
Visual and tactile sensation provided by the technique: Only 7 (26%) patients reported they were able to palpate the implant in some way. Three (13%) patients reported they could see the implant in their breasts (Figure 6).
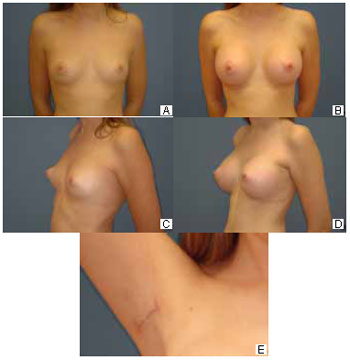
Figure 6 - A: Preoperative anterior view. B: Six-month postoperative previous view. C: Preoperative three-quarter view. D: Six-month postoperative three-quarter view. E: Axillary hollow at 6 months postoperatively.
DISCUSSION
Thin women, with a constricted lower pole, benefit the most from the partial submuscular insertion procedure, but this technique can also be used in those with dense glandular and thick fat tissue and those with mild-to-moderate ptosis2.
Silicone gel breast implant insertion by the axillary route using the partial submuscular location without videoendoscopic assistance involved the adequate surgical time for this type of procedure. It did not result in intraoperative complications apart from most other techniques for silicone gel implant insertion nor increased the length of hospitalization or changed the technique used by anesthesiologists. Although the muscle was dilated without direct viewing, no bruising or increased operative time was noted compared with the axillary and mixed plan techniques1,2,4-7.
The postoperative complications were similar to those associated with the subglandular or subfascial axillary technique4-6. Axillary contracture is the most frequent complication, followed by paresthesia or dysesthesia of the upper limb3. These complications have high incidence, but they rarely persist after 6 months; in addition, their incidence does not change with the use of videoendoscopy3-7. Axillary retraction is probably the result of lymphatic obliteration in the region or thrombophlebitis of Mordor in the armpit vessels3. These fibroids can pull the armpit nerves and cause paresthesia in the region. This hypothesis is supported by the simultaneous improvement of fiber strands and paresthesia in many cases3. Any type of strand, even temporary and not perceived by patients, was noted in the survey as a complication.
A major concern related to surgeries with axillary scar is alteration of the breast lymphatic drainage, which may be influenced by sentinel node testing and spread of breast neoplasms. The extent of the alteration of breast lymphatic drainage is not exactly clear in the literature because major studies on the sentinel node excluded patients with scars in the armpit. However, several recent studies have shown little interference of the scar in the manipulation of breast lymphatic drainage and development of mammary neoplasms8-10.
In relation to pain, the patients mostly classified the procedure as painful, but the pain was primarily located in the areas to be detached for the prosthesis, thus preventing long-term pain in the area for the axillary detachment. This fact is reinforced by the arm's easy mobilization in the first 2 weeks and early beginning of daily activities (2-3 weeks)4-7.
Most patients reported that the result was natural and that they failed to visualize the prosthesis, overcoming the preoperative fear of 74% of the patients that the implants would be visible. All patients stated that the muscle flap helped to camouflage the implants. Patients with poor coverage in the upper pole of the breasts are the biggest beneficiaries of this technique2. However, the muscle covers only one-third of the prosthesis, which is insufficient to prevent the prosthesis being felt.
Magnetic resonance imaging of the breast (Figure 7) and breast ultrasound (Figure 8) confirmed the muscle-based coverage of the upper-inner edge of the prosthesis, reinforcing the patients' view that the muscle flap was responsible for the most natural result. However, these results are hampered by the absence of a control group to compare the data and test their significance.
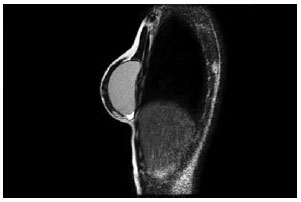
Figure 7 - Magnetic resonance image of an operated breast demonstrating pectoralis major interruption and coverage of the upper pole of the prosthesis.
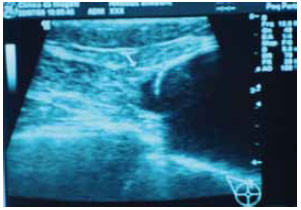
Figure 8 - Breast ultrasound image demonstrating the muscle coverage of the edge of the prosthesis (arrow).
CONCLUSIONS
Breast augmentation with silicone gel implants by the axillary route using the partial submuscular location without videoendoscopic assistance is a safe method that results in the expected complications and does not increase the associated morbidity.
The muscle dilation does not cause a considerable increase in the surgical time and ensures results widely appreciated by patients, without the need for too much detachment of the muscle from its costal insertion. The postoperative recovery is appropriate, without a change in its intensity or duration despite greater handling in the region. The patient satisfaction with the incision and partial submuscular location is very good, improving the implant upper-third coverage and offering an additional access route.
REFERENCES
1. Tebbetts JB. Axillary endoscopic breast augmentation: processes derived from 28-year experience to optimize outcomes. Plast Reconstr Surg. 2006;118(7Suppl):53S-80S.
2. Khan UD. Muscle-splitting breast augmentation: a new pocket in a different plane. Aesthetic Plast Surg. 2007;31(5):553-8.
3. Ghaderi B, Hoenig JM, Dado D, Angelats J, Vandevender V. Incidence of intercostobrachial nerve injury after transaxillary breast augmentation. Aesthetic Surg J. 2002;22(1):26-32.
4. Munhoz AM, Fells K, Arruda E, Montag E, Okada A, Aldrighi C, et al. Subfascial transaxillary breast augmentation without endoscopic assistance: technical aspects and outcome. Aesthetic Plast Surg. 2006;30(5):503-12.
5. Graf RM, Bernardes A, Auersvald A, Damasio RC. Subfascial endoscopic transaxillary augmentation mammaplasty. Aesthetic Plast Surg. 2000;24(3):216-20.
6. Momeni A, Padron NT, Bannasch H, Borges J, Björn Stark G. Endoscopic transaxilary subpectoral augmentation mamaplasty: a safe and predictable procedure. J Plast Reconstr Surg. 2006;59(10):1076-81.
7. Tebbetts JB. Dual plane breast augmentation: optimizing implant-soft-tissue relationship in a wide range of breast types. Plast Reconstr Surg. 2001;107(5):1255-72.
8. Munhoz AM, Aldrighi C, Ono C, Buchpiguel C, Montag E, Fells K, et al. The influence of subfascial transaxillary breast augmentation in axillary lymphatic drainage patterns and sentinel lymph node detection. Ann Plast Surg. 2007;58(2):141-9.
9. Huang GJ, Hardesty RA, Mills D. Sentinel lymph node byopsy in the augmented breast: role of the transaxillary subpectoral approach. Aesthet Surg J. 2003;23(3):184-7.
10. Munhoz AM, Aldrighi C, Buschpiegel C, Ono C, Montag E, Fells K, et al. The feasibility of sentinel lymph node detection in patients with previous transaxillary implant breast augmentation: preliminary results. 2005;29(3):163-8.
Plastic Surgeon at Hospital Bom Jesus de Ponta Grossa, Full member of the Brazilian Society of Plastic Surgery, Ponta Grossa, PR, Brazil.
Corresponding author:
Victor Mauro
Rua Carlos Osternack, 111 - 1º andar - Vila Estrela
Ponta Grossa, PR, Brazil - CEP 84010-120
E-mail: vicmauro@gmail.com
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Received: January 11, 2010
Paper accepted: February 5, 2011
Work performed at Hospital Bom Jesus de Ponta Grossa (Bom Jesus Hospital of Ponta Grossa), Ponta Grossa, PR, Brazil.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter