

Original Article - Year 2011 - Volume 26 -
Cranioplasty: parietal versus customized prosthesis
Cranioplastia: parietal versus prótese customizada
ABSTRACT
Introduction: Bone defects of the cranial vault often occur as a result of tissue loss due to trauma or to the treatment of tumors. Craniectomy performed by a neurosurgeon to treat cranioencephalic injury is the most common cause of post-trauma skull deformity. The main indications for the correction of skull defects include the protection of brain tissue; aesthetic correction; and clinical improvement, which includes soft tissue pulsation and feelings of insecurity reported by the patient. Extensive defects involving the full thickness of the skull pose a great challenge to the surgeon because of their problematic follow-up, which includes reports on previous surgeries, local infections, and osteonecrosis. This leads to difficulty in choosing the best reconstructive method. Methods: We present a retrospective analysis of cases of cranial calotte reconstruction operated on between January 2008 and April 2010 at the Centro de Cirurgia Crânio-maxilofacial do Instituto Nacional de Traumatologia e Ortopedia (MS, Brazil). Results: We analyzed 11 patients with extensive full-thickness skull defects. Nine of these patients underwent repair with autologous bone graft obtained from the outer table of the parietal bone, and 2 underwent repair with a customized prosthesis. Conclusions: According to protocol at our centre, the first method of choice for reconstructing large full-thickness skull defects is autologous graft using the outer table of the parietal bone without craniectomy. In selected cases in which this reconstructive method cannot be used, the use of a customized prosthesis is the best option.
Keywords: Skull. Craniocerebral Trauma. Parietal Bone. Prosthesis and Implants. Plastic Surgery.
RESUMO
Introdução: Os defeitos ósseos da calota craniana ocorrem frequentemente pela perda tecidual relacionada ao trauma ou para tratamento de tumores, sendo a maior causa de deformidade do crânio pós-trauma a craniectomia realizada na intervenção neurocirúrgica para tratamento da injúria cranioencefálica. As principais indicações para a correção dos defeitos cranianos incluem proteção do tecido cerebral, correção estética e melhora clínica, que envolve a pulsação de tecidos moles e a sensação de insegurança relatada pelo paciente. Defeitos extensos da calvária de espessura total são de grande desafio ao cirurgião pelo seu curso complexo, que envolve cirurgias prévias, infecções locais e osteonecrose, que leva à dificuldade na escolha do melhor método reconstrutivo. Método: Análise retrospectiva dos casos operados entre Janeiro de 2008 e Abril de 2010 para reconstrução da calota craniana realizados no Centro de Cirurgia Crânio-maxilofacial - Instituto Nacional de Traumatologia e Ortopedia (MS). Resultados: Foram analisados 11 pacientes com defeitos extensos de espessura total da calota craniana operados no período, destes 9 foram submetidos a correção com enxerto ósseo autólogo de tábua externa do parietal e 2 com prótese customizada. Conclusão: O protocolo do nosso centro define que a primeira opção de reconstrução para defeitos extensos da calota craniana de espessura total é o enxerto autólogo de tábua externa do osso parietal sem craniectomia. Nos casos selecionados em que esse método reconstrutivo não pode ser utilizado, a opção é pelo uso de prótese customizada.
Palavras-chave: Crânio. Traumatismos Craniocerebrais. Osso Parietal. Próteses e Implantes. Cirurgia Plástica.
Neurosurgery is often required for the acute-phase treatment of cranioencephalic trauma resulting from automobile accidents, falls, and interpersonal aggression; these are the most common etiological factors of acquired cranial defects1.
Cranioplasty for the correction of extensive cranial defects is particularly challenging for surgeons. Patients with such defects usually present with a complex clinical history that involves multiple previous operations; further, this procedure is technically difficulty and requires the appropriate materials and a skilled team to be successful2.
In cases of full-thickness loss of the cranial calotte, the main purpose of cranioplasty is to protect the brain and correct the apparent aesthetic deformity. Moreover, when defects are large enough to allow the scalp to exercise direct pressure against the brain, cranial reconstruction can result in an improvement in speech and in hemiparesis3 (Figure 1).
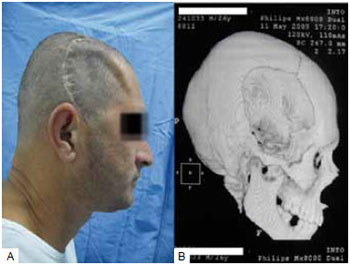
Figure 1 - Frontotemporal cranium defect. A: Frontotemporal defect; B: Computed tomography with 3-dimensional reconstruction.
Cranioplasty for the treatment of full-thickness loss of the skull includes various methods of reconstruction. Among the autologous materials used for this purpose are autologous grafts of the outer table of the parietal bone, ribs, and iliac crest. The choice of the donor area depends on several factors, including similarity with the tissue to be repaired in the defect area, ease of collection of the graft, proximity to the area to be reconstructed, and capacity for bone integration.
With the development of biomaterials, alloplastic materials have been used more widely in various areas of medicine. Several materials such as porous polyethylene, bioceramics, and titanium mesh are used for this purpose. The production of customized prostheses, which have dimensions and shapes that are appropriate for the correction of a given defect, allows for the increasing comprehensive use of alloplastic materials. Rapid prototyping in the health field involves producing a 3-dimensional model compatible with the anatomical structure that must be treated, obtained through information gathered from imaging studies (computed tomography). These prototypes, which reliably depict the defect, are used to produce customized prostheses, making the whole treatment process more efficient, simple, and accurate4.
METHODS
We performed a retrospective analysis of patients who underwent skull reconstruction between January 2008 and April 2010 at the Centro de Cirurgia Crânio-maxilofacial do Instituto Nacional de Traumatologia e Ortopedia (MS, Brazil).
Inclusion criteria were residual full-thickness defects that had resulted from neurosurgical treatment of cranioencephalic trauma, stroke, and tumors. Exclusion criteria were partial-thickness skull defects, bone tumors, and congenital malformations. Information was collected through survey data obtained from medical records and evaluation of preoperative computed tomography scans.
RESULTS
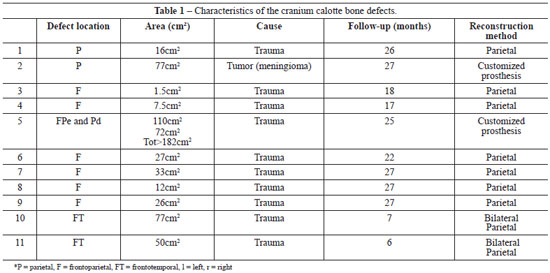
A total of 11 patients operated on during the period of interest were included and analyzed.
The full-thickness cranial defects analyzed had resulted from sequelae due to previous neurosurgical approaches. The most prevalent etiology was motorcycle accidents (9/11, 81.8%); there was also 1 case of interpersonal aggression and 1 case of tumor resection (meningioma).
The frontal and parietal regions were the most affected: the parietal region alone was compromised in 2 patients, the frontal region alone was compromised in 6 patients, the frontotemporal region was compromised in 2 patients, and left frontoparietal + right parietal in 1 patient.
The defect areas ranged in size from 1.5 cm2 to 182 cm2. The largest defect compromised the skull bilaterally (left frontoparietal region and right parietal region) and had a total area of 182 cm2 (Table 1).
In 9 cases, the reconstruction method chosen was the use of bone graft from the parietal outer table. In these cases, the defects were less than 77 cm2. We chose a coronal incision with an initial approach to the cranial area of the defect and, subsequently, the bone graft donor site at the parietal region, where the collection was made by removing the outer table of the parietal bone without craniectomy. The defect was corrected by fixing the graft with a rigid internal fixation material. At the end of the surgery, a pericranial flap rotation was performed to cover the reconstructed area. In the case of the 2 largest defects reconstructed with autologous parietal graft (50 cm2 and 77 cm2), we removed the parietal outer table bilaterally (Figures 2 and 3).
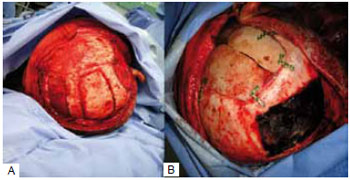
Figure 2 - Parietal graft for correction of extensive frontotemporal defect. A: Parietal donor of bilateral bone graft; B: Bone graft repairing the defect.
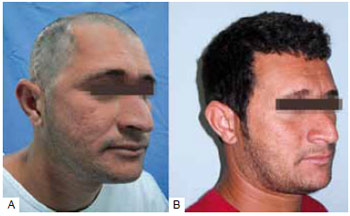
Figure 3 - Cranioplasty with parietal outer table bone graft. A: Preoperative; B: Six months postoperative.
In 2 cases, we opted to use a customized prosthesis to correct the cranial defect, and the alloplastic material used was bioceramics. In these cases, the defects reconstructed were larger than 77 cm2. Surgical access for placement of this material was made through a coronal incision, which allowed access to the defect area and subsequent placement and fixation of the customized prosthesis (Figure 4).
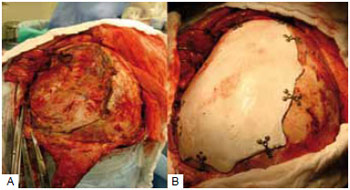
Figure 4 - Cranioplasty with a customized prosthesis. A: Area of the defect; B: The reconstructed defect.
Postoperative observation times ranged from 7 to 27 months, and none of the cases had postoperative infection or extrusion of the reconstruction material.
In 1 case, which was referred to us by another institution after the patient contracted a local infection due to a porous polyethylene prosthesis, we replaced this alloplastic material with an autologous parietal graft to control the infection.
DISCUSSION
The preferred approach for correcting small full-thickness skull defects is with the use of parietal outer table bone grafts. This type of reconstruction is indicated when the dimensions of the donor area are sufficient to correct the defect, the use of autologous grafts results in the various benefits discussed below, and the complications of this method are minimized by a limited donor collection area.
For treating large cranial calotte defects, the preference should be, whenever possible, the use of a parietal outer table bone graft2. In the cases treated in our center, defects smaller than 77 cm2 were adequately fixed using a parietal graft. In such cases, the dimensions of the donor area can be extended by the bilateral removal of the parietal bone graft.
The skull is the preferred donor site for the bone graft as compared to other donor sites such as the iliac crest and the ribs. Its main benefits are proximity to the area to be corrected; access through a single incision, which results in an aesthetic scar on the scalp; a graft with identical characteristics and embryological origin of the lost bone; and satisfactory volume and contour. At the donor area, the required thickness and rigidity of the internal cortical layer are maintained for cerebral protection; in addition, a visually acceptable scar outline is maintained1.
Good nutrition ensures the maintenance of this graft and minimizes the risk of infection and extrusion. Because this graft has a membranous origin, the surgical technique should ensure good bone contact by proper fixation and the routine use of the pericranial flap to cover the graft and increase nutrient intake. Thus, in cases of infection arising from the use of alloplastic materials, an ideal option is to replace the reconstructive material with bone grafts1.
In 1 of the cases in which a customized prosthesis was used, the defect was very large, making it impossible to cover the entire area of bone loss (182 cm2) with the bilateral parietal bone graft. We did not use autologous and alloplastic materials in this patient because of the possibility of introducing more complications.
In the second case in which we decided to use a customized prosthesis, the defect had similar dimensions to the parietal bone graft (77 cm2) but compromised the parietal region. This fact limited the possibility of expanding the donor area bilaterally using the parietal outer table.
In the cases studied here, the most commonly affected area was the frontal region (73%), which is consistent with the published data (53.2%). The parietal region alone was the second most affected site of the calvaria in our study (18%); this finding was in contrast with the findings of other reports, which place it in the third position after the temporal region5.
Evaluating bone loss in the calvaria is important not only for measuring the defect area but also for assessing the location of bone loss because the involvement of the parietal bone makes it impracticable for this region to be used as a donor area. This fact was true in both cases in which we chose to use a customized prosthesis.
The use of an autologous parietal outer table graft has apparent benefits for the patient, but in order to take advantage of them accurate indication for the use of the parietal bone graft is necessary.
In all cases, it is essential to properly assess the patient's history and perform a thorough physical examination so that the best reconstructive method can be determined.
Adult male patients have skulls with an average thickness of 0.7 cm, which is sufficient to perform detachment of the outer cortical layer of the parietal bone to be used as the bone graft. On the other hand, the thickness of the parietal bone is less in pediatric patients, elderly patients, and women, and in most cases cannot be used as a bone graft.
The evaluation of comorbidities during anamnesis is very important because the surgical risk increases with the collection of the parietal bone graft. There is increased blood loss due to the manipulation of the richly vascularized diploe, an approach required to remove the outer cortical layer of the cranial bone for the graft. The intensity of cranioencephalic injury depends on the thickness of the cranial vault, and treatment requires a very cautious approach. This surgical procedure takes longer as compared to the procedure for isolated placement of the customized prosthesis because of the additional time needed to remove the graft.
In cases when the use of a cranial graft is not practicable, the best option is the use of a customized prosthesis for reconstruction. After initial assessment and the indication of a customized prosthesis, computed tomography imaging and subsequent prototyping should be performed to ensure that the defect is analyzed as accurately as possible. The prosthesis is customized based on the prototype, allowing for complete defect correction.
In the health field, rapid prototyping has several benefits apart from the customization of prostheses. Before the surgery, it allows the surgeon to simulate the surgery and to evaluate the incision and fixation sites of the prosthesis; to compare the symmetry of the reconstruction with that of the healthy side; and to detect possible technical difficulties, reducing the length of the surgery. It also helps in establishing clearer and more effective communication with the patient about the procedure to be performed, ensuring greater satisfaction in the postoperative period6.
The most commonly used alloplastic materials for customization include bioceramics, titanium mesh, and methylmethacrylate, but there is no consensus in the present literature regarding the most suitable material in terms of extrusion and infection. Compared to bone grafts, alloplastic materials have the advantage of not being subject to resorption; however, the risks of infection and implant loss are more significant2,6-8.
CONCLUSION
According to the protocol at the Centro de Cirurgia Crânio- maxilofacial do Instituto Nacional de Traumatologia e Ortopedia (MS, Brazil), the reconstructive method for the treatment of full-thickness skull defects with a small area of bone loss should be the use of a parietal outer table bone graft.
In large full-thickness skull defects, it is essential to perform a careful evaluation of the patient with appropriate anamnesis and physical examination as well as detailed computed tomography imaging analysis to determine the best corrective method.
Even in cases with large calvarial defects, we prefer the use of a parietal outer table bone graft without craniectomy. To obtain all of the benefits of the method, when the cranial defect is very large, we expand the donor area bilaterally using the parietal donor.
In some cases, there are some limiting factors associated with the use of grafts, such as inappropriate dimensions, commitment of the parietal bone, inadequate thickness of the parietal bone, or associated comorbidities. In these selected cases, the best option is the use of a customized prosthesis.
REFERENCES
1. Sahoo N, Roy ID, Desai AP, Gupta V. Comparative evaluation of autogenous calvarial bone graft and alloplastic materials for secondary reconstruction of cranial defects. J Craniofac Surg. 2010;21(1):79-82.
2. Zins JE, Moreira-Gonzalez A, Papay FA. Use of calcium-based bone cements in the repair of large, full-thickness cranial defects: a caution. Plast Reconstr Surg. 2007;120(5):1332-42.
3. Yaremchuck MJ. Acquired cranial bone deformities. In: Mathes SJ, Hentz VR, eds. Plastic surgery. 2nd ed. Philadelphia:Elsevier;2006. p.547-62.
4. Chim H, Schantz JT. New frontiers in calvarial reconstruction: integrating computer-assisted design and tissue engineering in cranioplasty. Plast Reconstr Surg. 2005;116(6):1726-41.
5. Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: critical review in long-term followup. J Craniofac Surg. 2003;14(2):144-53.
6. Cao D, Yu Z, Chai G, Liu J, Mu X. Application of EH compound artificial bone material combined with computerized three-dimensional reconstruction in craniomaxillofacial surgery. J Craniofac Surg. 2010;21(2): 440-3.
7. Abuzayed B, Tuzgen S, Canbaz B, Yuksel O, Tutunculer B, Sanus GZ. Reconstruction of growing skull fracture with in situ galeal graft duraplasty and porous polyethylene sheet. J Craniofac Surg. 2009; 20(4):1245-9.
8. Chao MT, Jiang S, Smith D, DeCesare GE, Cooper GM, Pollack IF, et al. Demineralized bone matrix and resorbable mesh bilaminate cranioplasty: a novel method for reconstruction of large-scale defects in the pediatric calvaria. Plast Reconstr Surg. 2009;123(3):976-82.
1. Member of the Brazilian Society of Plastic Surgery (SBCP); Trainee at the Cranium-Maxillo-Facial Surgery Center of the National Institute of Traumatology and Orthopaedics - MS, Rio de Janeiro, RJ, Brazil.
2. Full Member of the SBCP; Coordinator of the Cranium-Maxillo-Facial Surgery Center of the National Institute of Traumatology and Orthopaedics - MS, Rio de Janeiro, RJ, Brazil.
Corresponding author:
Tatiana Hara
Rua Dr. Moretz Shons, 690 / 21A - Vila Guimarães
São Paulo, SP, Brazil - CEP 13630-170
E-mail: tatianahara@yahoo.com.br
Submitted to SGP (Sistema de Gestão de Publicações/Manager Publications System) of RBCP (Revista Brasileira de Cirurgia Plástica/Brazilian Journal of Plastic Surgery).
Received: October 21, 2010
Accepted: February 5, 2011
Work was performed at the Centro de Cirurgia Crânio-maxilofacial do Instituto Nacional de Traumatologia e Ortopedia (INTO) - (Cranium-Maxillo-Facial Surgery Center of the National Institute of Traumatology and Orthopaedics - INTO), Rio de Janeiro, RJ, Brazil.
Work was presented at the 47th Brazilian Congress of Plastic Surgery, Vitória, ES, Brazil, 2010; Winner of the Ivo Pitanguy Award.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter