

Original Article - Year 2025 - Volume 40Issue 1
Mastopexy Associated with Silicone Implants: Implant Support Technique with a Half-Cup Bra-Type Inferior Fascial Flap
Mastopexia associada a implantes de silicone: Técnica de sustentação dos implantes com retalho fascial inferior tipo "sutiã meia-taça"
ABSTRACT
Introduction Mastopexy with silicone implants is one of the most common cosmetic breast surgeries. Implant stabilization is a fundamental factor in avoiding early recurrence of ptosis. This study describes an implant-support technique in which a fascial flap of the pectoralis major muscle is extended inferiorly with the rectus abdominis and external oblique fascia, and laterally with serratus fascia, to simulate a half-cup bra. It also outlines the indications and outcomes for this method.
Materials and Methods This prospective study included 37 patients with breast ptosis who underwent the described technique from March 2021 to March 2023.
Results We used three implant surfaces: microtextured, textured, and polyurethane. The outcomes were very good and excellent in 92.5% of the cases. Epidermolysis was the most frequent complication and occurred in 12.5% of the patients, or 9.4% of the operated breasts.
Conclusion The distal flap of the deep mammary fascia, including its inferior and lateral continuation with the fasciae of the adjacent muscles, covering the lower half of the implant, proved to be an effective support. We indicate this flap for patients who want less "artificial" outcomes, with a defined breast but moderate cleavage projection. The technique presented a low incidence of complications.
Keywords: breast implantation; breast implants; mammaplasty; surgery, plastic; surgical flaps; surgical procedures, elective; surgical procedures, operative
RESUMO
Introdução A mastopexia com inclusão de implantes de silicone é uma das cirurgias estéticas mais comuns das mamas. A estabilização dos implantes é um fator fundamental para evitar recidiva precoce da ptose mamária. Nesta pesquisa descrevemos uma técnica de sustentação dos implantes através de um retalho da fáscia mamária profunda do músculo peitoral maior que se estende inferiormente na fáscia do músculo reto abdominal e obliquo externo e lateralmente coma fáscia do serrátil, mimetizando um sutiã meia-taça. Também foram apresentadas as indicações e resultados desse método.
Materiais e Métodos Foi realizado uma pesquisa prospectiva dos resultados obtidos com a técnica descrita, utilizada em 37 pacientes com flacidez mamária, operadas de março de 2021 a março de 2023.
Resultados Foram utilizadas três superfícies de implantes: microtexturizados, texturizados e de poliuretano. Os resultados foram classificados como muito bom e excelente em 92,5% dos casos. Epidermólize foi a complicação mais frequente, ocorrendo em 12,5% das pacientes ou 9,4% das mamas operadas.
Conclusão O retalho distal da fáscia mamária profunda, incluindo sua continuação inferior e lateral comas fáscias dos músculos adjacentes, cobrindo ametade inferior do implante, mostrou-se eficaz para sustentação. É indicado para pacientes que desejam resultados menos "artificiais", com a mama definida, porém com projeção moderada do colo mamário. A técnica apresentou baixa incidência complicações.
Palavras-chave: cirurgia plástica; implante mamário; implantes de mama; mamoplastia; procedimentos cirúrgicos eletivos; procedimentos cirúrgicos operatórios; retalhos cirúrgicos
Introduction
Breast sagging occurs when the skin coverage becomes excessive about the breast volume, resulting in a disproportionate relationship between the content and the container. This is very common after pregnancy, due to skin stretching from increased breast volume during lactation. The most recommended plastic surgery for correcting this condition is mastopexy. Additionally, this procedure is often sought by women who have suffered significant weight loss and by those showing sagging since the beginning of breast development.
Breast sagging may be associated with hypomastia or breast hypertrophy. Volume increase uses implants and adjusts the excessive skin in one or two surgical times. In patients with breast volume and sagging who wish for firm breasts and a marked upper pole as aesthetic results, similar to breast augmentation, the preferred technique is partial resection of the volume, silicone implant placement, and mastopexy. In both cases, the literature shows a greater possibility of complications after breast augmentation and mastopexy during the same surgical time. However, current technical advances, such as mastopexy with silicone implant placement (augmentation mastopexy), have been preferred over the two-stage surgery.1,2
A frequent cause of dissatisfaction with mastopexy with silicone implant placement is the early recurrence of breast ptosis with upper pole effacement.3,4 Several techniques aim to solve this issue by using tissue structures for implant support, such as the dermoglandular pedicle,5 the deep mammary fascia,6 the pectoralis major muscle7,8 and mixed techniques with more than one structure.9
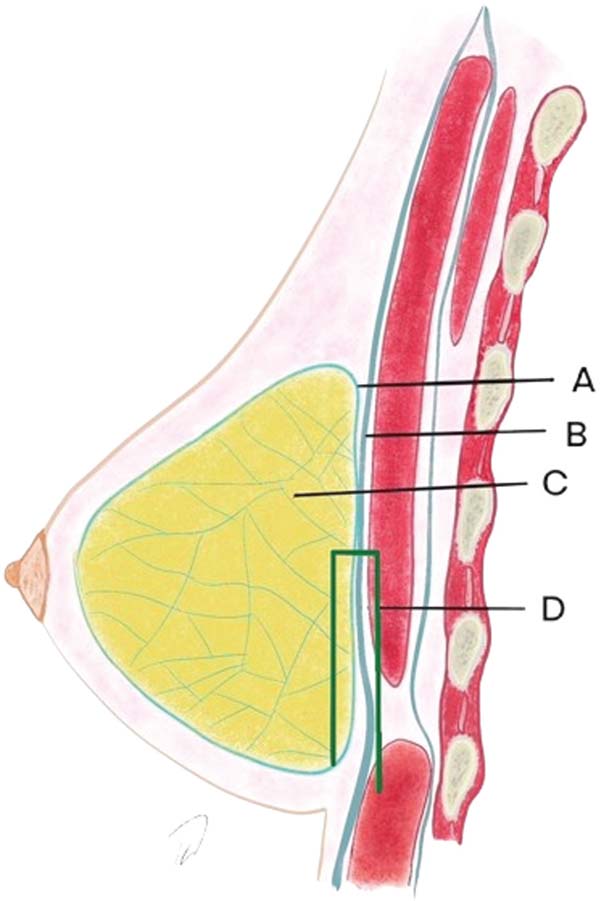
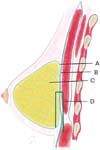
Graf et al. were the first authors to propose using the subfascial plane for breast implant placement, in 199910 and 2003.11 The pectoralis major or deep fascia consists of dense connective tissue, with an average thickness of 0.49 mm in the upper part, 0.60 mm in the lower, 0.52 mm in the medial, and 0.68 mm in the lateral part.12 The tissue continues over the adjacent rectus abdominis, serratus anterior, and external oblique muscles.
Furthermore, the deep mammary fascia presents some firm connection points with the deep layer, which are interesting to spare and not detach during the subfascial dissection, as they are above this plane. These three points are the following: close to the second rib; at the level of the fourth intercostal space; and in the inframammary fold.13 As a firm and inelastic structure, it can be an option for stabilizing implants in augmentation mastopexy.14
Objective
The present study aimed to describe a technique for mastopexy with silicone implant placement using the deep fascia for implant support and stabilization, to mimic a half-cup bra. We also evaluated outcomes in a prospective follow-up 1 year after surgery, regarding complications and level of satisfaction.
Materials and Methods
The current prospective study was conducted at Clínica Spani Vendramin from February 2021 to August 2024. It complied with the principles of the Declaration of Helsinki, and it received authorization from the Research Ethics Committee of the Institute of Health Sciences, Universidade Federal do Pará (CAAE: 81283224.8.0000.0018).
The study describes a mastopexy technique with silicone implant placement supported by a distal deep fascia flap.
The first author performed the surgeries from March 2021 to March 2023 using the technique described here. The minimum follow-up period was 1 year. We collected information on the degree of ptosis per the Regnault classification,15 implant type and volume, complications, and level of satisfaction. The assessment of this last item happened at least 1 year after surgery using a 5-point Likert scale, in which 1 ¼ poor satisfaction, 2 ¼ reasonable, 3 ¼ good, 4 ¼ very good, and 5 ¼ excellent satisfaction.
The inclusion criteria were patients aged 18 to 60 years, with breast ptosis, undergoing mastopexy with silicone implant placement using the technique herein described. The exclusion criteria were smoking patients, with a body mass index (BMI) above 30 kg/m2 or body fat percentages above 40%, and subjects not available for follow-up.
Planning
A previous medical consultation led to the indication of mastopexy with silicone implant placement supported by a fascial flap to patients who wanted more natural outcomes, a slight to moderate upper pole projection, no evolution to a waterfall appearance (high implant with ptosis of the breast parenchyma), and no exaggerated breast movement during pectoral muscle contraction.
The surgeon and the patient chose the implant together, including its type, surface, volume, and brand. The final choice considered the patient’s preferences, measurements of the chest and breast base, and the advantages and disadvantages of each type of implant. The choice of mastopexy technique (vertical, L-shaped, or inverted T-shaped) prioritized the smallest scars, as long as treating excessive skin was feasible.
Preoperative Marking
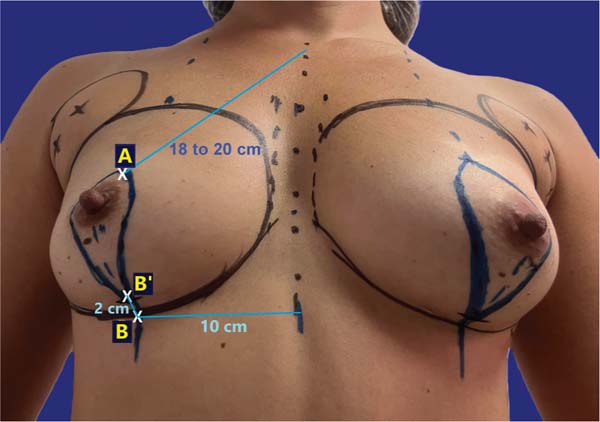
The surgeon marked point A on the projection of the inframammary fold (IMF) at the breast midline, about 18 to 20 cm from the sternal notch, and point B on the IMF, approximately 10 cm from the body midline. Then, the surgeon marked point B’ on the breast midline, 2 cm above B, and drew two curved lines from A to B’, one passing medially and the other laterally to the areola, to form a skin spindle (►Fig. 1). This spindle acted as a provisional guideline for tissue excision, as final confirmation occurred intraoperatively after implant placement.
Surgical Technique
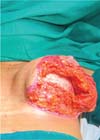
The patients underwent surgery with epidural anesthesia and sedation or general anesthesia. The procedure began with breast contour liposuction on the lateral part of the chest and anterior part of the armpit. This fat was set aside for potential refinement at the end of the surgery. Then, the surgeon performed a periareolar decortication, followed by an incision in the breast tissue at the lower pole, along the margins of the marking, up to approximately 0.5 cm above the muscle fascia. This dissection plane was maintained throughout the lower pole of the breast, that is, the site of flap creation. This thin layer of breast tissue left over the fascia helps with manipulating the fascial flap during its dissection. The surgeon removed the required amount of tissue from the lateral and central breast, leaving them with a thickness of approximately 3 to 4 cm, as well as from the medial portion of the breast, leaving it with 2.5 to 3 cm in thickness.
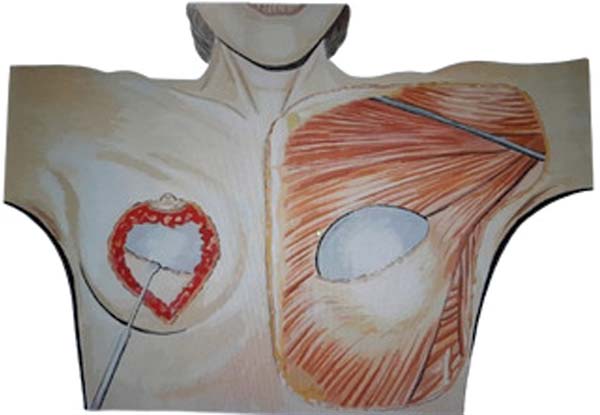
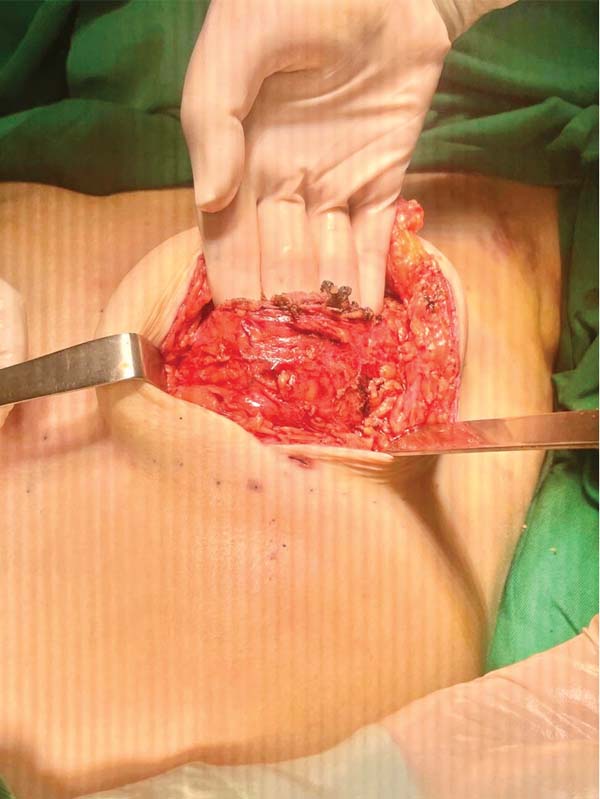
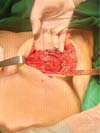
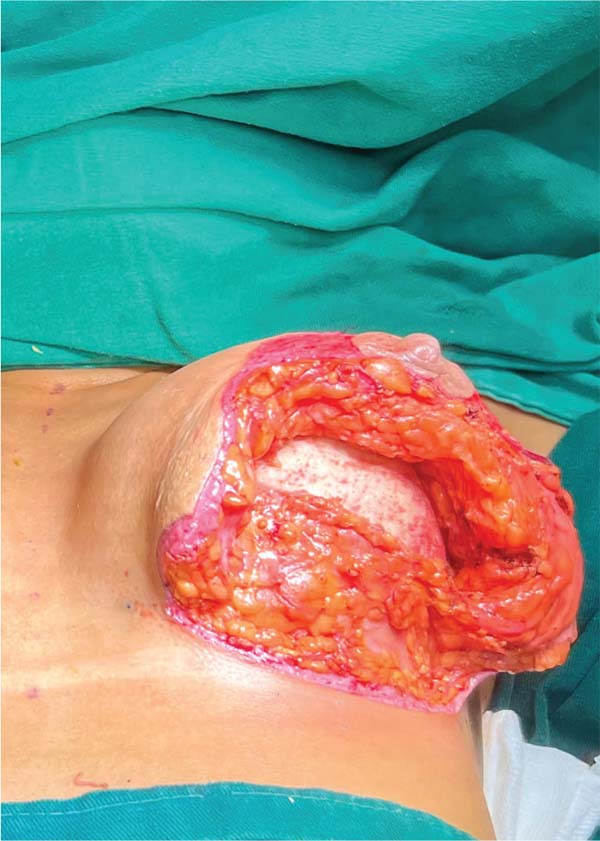
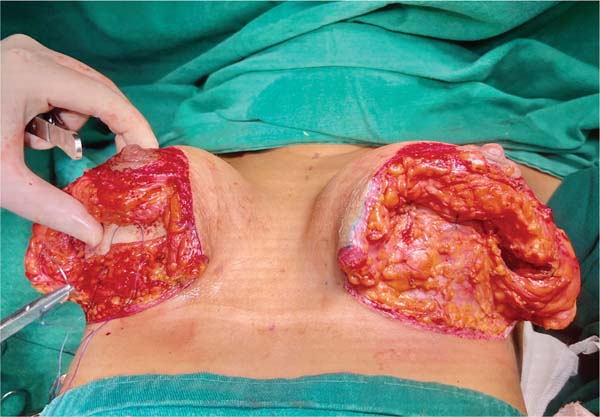
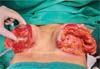
Next, the surgeon made an oblique incision in the muscular fascia at the level of the fourth rib, dissecting the fascial flap from the proximal to the distal region, until reaching the level of the SFM (►Fig. 2). The flap consisted of muscular fascia, a deep portion of the superficial mammary fascia, and a thin layer of mammary parenchyma (►Fig. 3). To avoid fascial sectioning during flap dissection, the surgeon directed the tip of the electric scalpel slightly toward the muscle and carefully pulled the fascia upward and forward, tilting the retractor. At the end of the dissection, the flap looked like a pouch (►Fig. 4). After rinsing the detached area with saline solution and changing gloves, the implant was inserted, with the lower half under the fascial flap and the upper half in the subglandular plane. As such, the support provided by the flap was similar to a half-cup bra (►Fig. 5).
The surgeon adjusted the skin by pulling the medial edge toward the lateral side and resecting the excess, using point B as the breast’s meridian reference. Then, the surgeon pulled the lateral edge toward the medial side, resecting the excess, using the same reference point. The surgeon sutured the fascial flap in the deep, upper portion of the breast parenchyma, applying cephalic traction, with three to four X- shaped stitches of 2.0 polyglactin thread (►Fig. 6). At this point, the surgeon assessed the firmness of the fascial tissue by pushing the implant superiorly and medially.
The surgeon positioned the nipple-areola complex (NAC) at point A, loosening it on the lateral and medial portions just enough to accommodate it without tension. The breast tissue and deep dermis suture used 2.0 and 3.0 polyglactin threads, respectively. After raising the upper part of the surgical table to leave the patient seated, the surgeon marked the NAC position, leaving a vertical line of around 6 cm, and finishing the skin adjustment in the horizontal direction in an L- shaped or inverted T-shaped, as needed, and performed the intradermal suture with 4.0 polyglecaprone thread.
Results
During the study, 37 patients underwent surgery using this technique, following the inclusion and exclusion criteria. As 5 patients were not available for follow-up for up to 1 year after surgery, the sample consisted of 32 subjects. The patient with the shortest follow-up time was 1 year, and the one with the longest follow-up time was 2 years and 6 months. Ages ranged from 18 to 56 (mean: 41.7) years; BMI ranged from 22.5 to 28.8 (mean: 27.1) kg/m2; and body fat percentage (BFP) ranged from 23.3 to 37.2%.
There were 15 patients with grade III ptosis, 6 with II ptosis, and 11 with grade I. Implant volumes ranged from 250 to 430 mL, with a median volume of 305 mL. Also, 10 patients used high-profile conical polyurethane implants (Silimed Advance HI) and 22 used round implants distributed as follows: 3 high-profile polyurethane implants (Silimed Maximum HI), 5 textured, high-profile implants (Silimed Maximum HI, True texture), 7 extra-projection profile polyurethane implants (Polytech Meme XP, Microthane), 6 extra-projection profile microtextured implants (Polytech Meme XP), and 1 extra-projection profile with expanded silicone foam coating (LifeSil Adherence BEJ), as shown in ►Tables 1-2.
| Silicone implant shape | Number of cases | Percentage |
|---|---|---|
| Conical | 10 | 31.25% |
| Round, high profile | 8 | 25.00% |
| Round, extra-projection profile |
14 | 43.75% |
| Total | 32 |
| Silicone coating | Number of cases | Percentage |
|---|---|---|
| Polyurethane | 20 | 62.50% |
| Expanded silicone foam | 1 | 3.12% |
| Texturized | 5 | 15.62% |
| Microtexturized | 6 | 18.75% |
| Total | 32 |
| Complications | Number of cases | Percentage |
|---|---|---|
| Epidermolysis at scar junction or at the nipple-areola complex | 4 | 12.5% |
| Enlarged scar | 2 | 6.25% |
| Dyschromic scar | 2 | 6.25% |
| Hypertrophic scar | 1 | 3.12% |
| Infection | 0 | 0 |
| Seroma | 0 | 0 |
| Implant dislocation | 0 | 0 |
| Early recurrence of ptosis | 0 | 0 |
| Double bubble | 0 | 0 |
| Cleavage asymmetry | 0 | 0 |
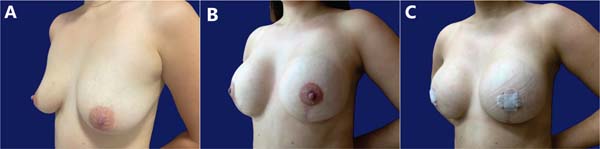
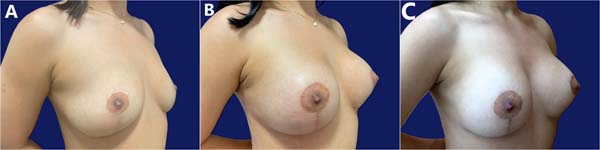
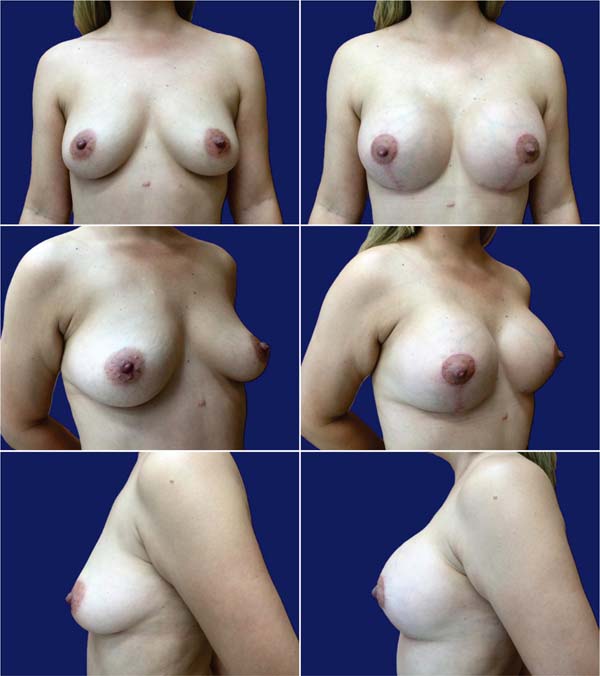
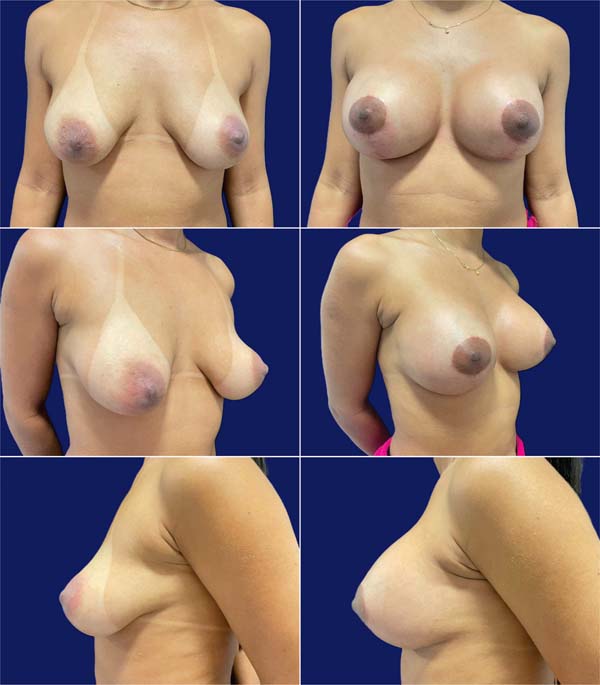
Table 3 describes complications. Epidermolysis was the most frequent one, occurring in 4 patients (12.5%), including two bilateral and two unilateral cases, with 6 cases in 64 breasts (9.4%). There was no recurrence of ptosis during this period, with little change in the outcome over time (►Figs. 7-8).
Most (92.5%) of the patients considered the outcome very good or excellent (►Figs. 9-10), and two classified the outcomes as good. One of them had a dehiscence widening the scar, requiring a touch-up under local anesthesia 1 year after the index procedure, which satisfied the patient. The other had a hyperchromic scar and received a referral for treatment with a dermatologist. This patient classified the outcome as reasonable and underwent several treatments, including occlusive steroid (Drenison) dressing, triamcinolone cream 1%, tamoxifen cream 1%, silicone plate, and microneedling. These treatments improved the scar and there was no indication for corrective surgery. The patient reported satisfaction with scar improvement and ultimately deemed the overall outcome satisfactory.
Discussion
Plastic surgeons have a high demand for mastopexy with silicone implants in patients with sagging breasts after pregnancy, bariatric surgery, significant weight loss, or specific breast conditions with abnormal development since puberty. Our patients were aged between 18 and 56 (mean: 41.7) years.
The patients selected for this technique were more reserved and discreet, did not like to draw too much attention to themselves, and did not want a large breast enhancement or an obvious appearance of having implants. Regarding the implant shape, we recommended the conical model for patients seeking more natural outcomes and round implants, with a high profile or extra-projection for those who desired a moderately defined cleavage. For patients who wanted a very highlighted cleavage, we indicated another type of surgery, with support provided by a muscular loop. These subjects were not included in this study.
Regarding coating, the most preferred implant was polyurethane (62.5%), as it added implant stabilization. However, 18.75 and 15.6% received microtextured and textured implants, respectively, with no outcome impairment, demonstrating the ability of the fascial flap to support these three types of implants.
Initially, mastopexy surgeries with silicone implant placement were performed in the subglandular plane, with implant positioning in the Chassaignac space, often destroying the deep connections of the superficial mammary fascia.12,13 As a result, the implant weight fell on the skin, which soon became loose, leading to early recurrence of ptosis, with a high complaint rate.16 This phenomenon led to the development of techniques to provide firmer structures for implant support, preventing it from exerting pressure on the skin.
Many tactics using muscle tissue, such as muscle loop, double plane, and total submuscular approaches, have been described with good outcomes in terms of implant support.2,7,8 However, submuscular techniques have some disadvantages, including implant displacement, widening of the space between the breasts, less control of the superomedial pole filling, distortion of the breast shape due to pectoral muscle contraction, and higherpossibility of asymmetry,mainly at the cleavage.1,4
Moreover, surgeries with muscle support are usually more complex and prone to waterfall, especially if a slightly larger volume of breast tissue remains. Excessive movement during contraction of the pectoralis major muscle is another feature of this surgery that patients do not like, especially those who want more natural outcomes. The use of glandular flaps to support the implants has not been very successful in preventing ptosis,16 as the superficial mammary fascia is not strong enough to support the implant. However, the deep mammary fascia, corresponding to the muscular fascia, has different characteristics, as it consists of dense connective tissue, which is firmer and inelastic.10-13
The use of muscle fascia may be an interesting option for this type of patient, with long-lasting results (►Fig. 7). Our technique is similar to the composite reverse inferior muscle sling (CRIMS) described by Munhoz et al.,17 but we use a fascial flap instead of a muscle one. Some patients have very thin fascia. In these cases, the surgical tactic was changed to use a muscle loop or dissect an intramuscular flap, leaving fibers above and below the detachment plane.
The technique described here led to a low complication rate, consistent with most mastopexy studies.2,6,7,18 One case required a scar retouch under local anesthesia and, after 1 year of follow-up, no patient was dissatisfied with the shape of their breasts or complained of implant ptosis that would suggest the need for reoperation. This high level of satisfaction results from screening during the preoperative consultation, aligning patients’ expectations with reality, and correctly selecting subjects who want more natural outcomes or a defined upper pole, but without exaggeration. The fascial flap would also help young patients who may want to breastfeed in the future and, therefore, should not undergo excessive breast tissue removal. In these cases, a submuscular implant could lead to a waterfall appearance and the fascial flap would reduce this possibility.
Early recurrence of breast ptosis usually occurs within the first year. In the current study, patients’ follow-up ranged from 1 year and 2 months to 2 years and 6 months. However, continued follow-up is critical to ensure the maintenance of outcomes for longer periods.
Conclusion
This mastopexy with silicone implant placement technique had good outcomes during the follow-up period of up to 2.5 years, with a low incidence of complications. The distal fascial flap, designed like a half-cup bra, is safe and efficient to support implants, whether polyurethane-coated, textured, or microtextured. The technique is a good option, especially for patients who seek implants marking the upper pole mildly to moderately.
References
1. Artz JD, Tessler O, Clark S, Patel S, Torabi R, Moses M. Can It Be Safe and Aesthetic? An Eight-year Retrospective Review of Mastopexy with Concurrent Breast Augmentation. Plast Reconstr Surg Glob Open 2019;7(06):e2272. Doi: 10.1097/GOX.0000000000002272
2. Procópio LD, Silva DDP, Rosique R. Implante submuscular em duplo bolso para mastopexias de aumento. Rev Bras Cir Plást 2019;34(02):187-195. Doi: 10.5935/2177-1235.2019RBCP0133
3. Khavanin N, Jordan SW, Rambachan A, Kim JYS. A systematic review of single-stage augmentation-mastopexy. Plast Reconstr Surg 2014;134(05):922-931. Doi: 10.1097/PRS.000000000000 0582
4. Neves LJVA. Reoperações após mamoplastias redutoras e mastopexias associadas a implantes de silicone. Rev Bras Cir Plást 2019; 34(Suppl 2):79-82. Doi: 10.5935/2177-1235.2019RBCP0126
5. Pessoa MCM, Accorsi AJ Jr, Ribeiro L, Moreira LF. Mastopexia com implantes: uso sistemático dos retalhos de base inferior de Ribeiro. Rev Bras Cir Plást2013;28(03):333-342.Available from: https://rbcp.org.br/details/1417/pt-BR/mastopexia-comimplantes-uso-sistematico-dos-retalhos-de-base-inferior-deribeiro
6. Graça Neto L. Tratamento da ptose mamária através da colocação de implantes de silicone subfascial seguidos de mastopexia em “T” invertido. Rev Bras Cir Plást 2020;35(03):269-275. Doi: 10.5935/2177-1235.2020RBCP0049
7. Ono MT, Karner BM. Four-step Augmentation Mastopexy: Lift and Augmentation at Single Time (LAST). Plast Reconstr Surg Glob Open 2019;7(11):e2523. Doi: 10.1097/GOX.0000000000002523
8. Rigo MH, Piccinini PS, Sartori LDP, Carvalho LARd, Uebel CO. SMSSplit Muscle Support: A Reproducible Approach for Breast Implant Stabilization. Aesthetic Plast Surg 2020;44(03):698-705. Doi: 10.1007/s00266-019-01565-5
9. Araujo LRR, Itikawa WM. Técnica de duplo espaço modificada para mastopexia de aumento. Rev Bras Cir Plást 2022;37(01): 36-44. Doi: 10.5935/2177-1235.2022RBCP0007
10. Graf RM, Bernardes A, Auersvald A, Damasio RCC. Mamaplastia de aumento transaxilar videoendoscópica subfascial. Rev Bras Cir Plást 1999;14(02):45-54. Available from: https://www.rbcp.org.br/details/214/pt-BR/mamaplastia-de-aumento-transaxilar-videoendoscopica-subfascial
11. Graf RM, Bernardes A, Rippel R, Araujo LR, Damasio RC, Auersvald A. Subfascial breast implant: a new procedure. Plast Reconstr Surg 2003;111(02):904-908. Doi: 10.1097/01.PRS.0000041601. 59651.15
12. Jinde L, Jianliang S, Xiaoping C, Xiaoyan T, Jiaqing L, Qun M, Bo L, et al. Anatomy and clinical significance of pectoral fascia. Plast Reconstr Surg 2006;118(07):1557-1560. Doi: 10.1097/01. prs.0000237002.89761.98
13. Rehnke RD, Groening RM, Van Buskirk ER, Clarke JM. Anatomy of the superficial fascia system of the breast: a comprehensive theory of breast fascial anatomy. Plast Reconstr Surg 2018;142 (05):1135-1144. Doi: 10.1097/PRS.0000000000004948
14. Graf R. Does fascia provide additional, meaningful coverage over a breast implant? Plast Reconstr Surg 2004;113(02):777- 779; author reply 779-780. Doi: 10.1097/01.PRS.0000104516. 13465.96
15. Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg 1976;3(02):193-203. Doi: 10.1016/S0094-1298(20)30220-0
16. Gomes RS. Mastopexia com retalho de pedículo superior e implante de silicone. Rev Bras Cir Plást 2008;23(04):241-247. Available from: https://rbcp.org.br/details/380/pt-BR/mastopexia-com-retalho-de-pediculo-superior-e-implante-desilicone
17. Munhoz AM, Marques Neto A, Ferrari O. Single-stage augmentation mastopexy with composite reverse inferior muscle sling technique for autologous reinforcement of the inferior pole: technical refinements and outcomes. Aesthet Surg J 2020;40 (06):NP356-NP373. Doi: 10.1093/asj/sjz334
18. Sodré RL, Calil JA, Ferreira RA, do Espírito SantoPRQ,Ruiz CT, Piotrowsky MF, et al. A mastopexia de aumento em L. Rev Bras Cir Plást 2023;38(02):e0684. Doi: 10.5935/2177-1235.2023 RBCP0684-EN
1. Universidade Federal do Pará, Belém, PA, Brasil
2. Clínica Spani Vendramin, Belém, PA, Brasil
3. Medicine Program, Universidade Federal do Pará, Belém, PA, Brasil
4. Medicine Program, Centro Universitário Metropolitano da Amazônia, Belém, PA, Brasil
Address for correspondence Fabiel Spani Vendramin, Universidade Federal do Pará, Belém, PA, Brasil (e-mail: drfabiel@gmail.com).
Artigo submetido: 29/09/2024.
Artigo aceito: 20/05/2025.
Conflict of Interests
The authors have no conflict of interests to declare.










 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter