

Original Article - Year 2024 - Volume 39 -
Tranexamic Acid in the Incidence of Hematoma in Breast Explantation Surgery
Ácido tranexâmico na incidência de hematoma na cirurgia de expiante mamário
ABSTRACT
Introduction Surgical procedures are subject to several postoperative complications and hematoma is a frequent occurrence. Surgeries involving wide dissection, such as total intact capsulectomies, are more prone to bleeding. Several medications have been used to reduce hematomas, such as tranexamic acid. No articles in the medical literature analyze the relationship between tranexamic acid and the incidence of hematomas in total intact capsulectomy surgeries.
Methods This study collected retrospective medical record data to determine the incidence of hematoma in total intact capsulectomy surgeries in two groups of patients. Patients underwent total intact capsulectomy with or without mastopexy and there was no insertion of a new breast implant. A group of 140 patients received intravenous and topical tranexamic acid during surgery and another group of 140 patients did not. Surgeries occurred from January 2022 to December 2023 and data subsequently underwent statistical analysis.
Results The two groups were comparable and presented normal distribution. Tranexamic acid statistically reduced the incidence of hematoma (p value = 0.004). Implant size was also statistically significant in decreasing hematoma incidence, with a mean volume of 350 mL in cases with hematoma and 291 mL in the group without hematoma (p value = 0.020). The remaining variables presented p values <0.05.
Conclusion Topical and intravenous use of tranexamic acid reduces the incidence of hematoma after intact total capsulectomy in patients with breast implants.
Keywords: breast; mammoplasty; breast implants; postoperative complications; hematoma
RESUMO
Introdução Procedimentos cirúrgicos estão sujeitos a diversas complicações no pósoperatório, sendo o hematoma um dos mais frequentes. Cirurgias que apresentam dissecção ampla, como as capsulectomias totais intactas, estão mais sujeitas a sangramentos. Diversas medicações têm sido utilizadas na diminuição dos hematomas, como o ácido tranexâmico. Não há na literatura médica artigos analisando a relação do ácido tranexâmico na incidência de hematomas nas cirurgias de capsulectomia total intacta.
Método Estudo retrospectivo em que foi levantado dados de prontuário para determinar a incidência de hematoma nas cirurgias de capsulectomia total intacta em dois grupos de pacientes. As pacientes foram submetidas a capsulectomia total intacta com ou sem mastopexia, não foi realizado nova inclusão de implante mamário. Um grupo de 140 pacientes recebeu ácido tranexâmico intravenoso e tópico durante a cirurgia e outro grupo de 140 pacientes não recebeu a medicação. Os dados foram coletados entre janeiro de 2022 e dezembro de 2023 e submetidos à análise estatística.
Resultados Os grupos foram considerados comparáveis e com distribuição normal. Foi verificado uma significância estatística do uso do ácido tranexâmico na diminuição de incidência de hematoma com valor de p = 0,004. Existe significância estatística para o tamanho do implante, a média ficou em 350ml para os casos com hematoma contra 291 ml no grupo sem hematoma (valor de p = 0,020). Não houve associação ou p < 0,05 para as demais variáveis estudadas.
Conclusões O uso tópico e intravenoso de ácido tranexâmico reduz a incidência de hematoma no pós-operatório de cirurgias que envolvem capsulectomia total intacta em pacientes com implante mamário.
Palavras-chave: mama; mamoplastia; implante mamário; complicações pós-operatórias; hematoma
In addition to good hemostasis, surgeons have studied other ways to reduce the incidence of hematoma. Surgical drains do not prevent arterial hematoma but reduce the incidence of seroma and can solve small-volume venous hematoma.4
Several medications have been used to reduce the incidence of hematoma. Tranexamic acid has been increasingly used to decrease the incidence of hematoma and seroma, blood loss, and the need for blood transfusions without elevating the risk of thromboembolic events.5,6,7,8
Hemostasis is a balance between fibrinolysis and the coagulation cascade. In tissue injury, the coagulation cascade produces thrombin, which converts fibrinogen into fibrin and stabilizes platelets. The fibrinolysis cascade causes lysine to bind to plasminogen receptors, activating them into plasmin, leading to fibrin degradation and platelet activation. This system prevents one cascade from overriding the other. However, in the postoperative period, temporary fibrinolysis suspension reduces bleeding.9,10,11
Patented in 1957, tranexamic acid is a synthetic lysine analog that competitively blocks plasminogen receptors and inhibits their tissular activation. Plasminogen receptor blockade prevents their activation into plasmin and, as a result, the lysis of fibrin polymers. Additionally, tranexamic acid reduces platelet consumption and acts as an antiinflammatory since plasmin has several inflammatory effects.3,9
Randomized studies using tranexamic acid have observed decreased intraoperative bleeding in surgeries such as rhinoplasty, rhytidoplasty, liposuction, and reduction mammoplasty. Topical use of tranexamic acid reduced drain output in reduction mammoplasty.3
In recent years, there has been an increase in breast implant removal surgeries, popularly known as explantation but better described as total intact capsulectomy. These surgeries involve a large detachment of the breast to remove the entire capsule surrounding the implant. Such detachment can favor the appearance of hematomas, especially if the implant is under the muscle.12,13,14
Although there are studies on tranexamic acid in several plastic surgery procedures, the literature has no articles regarding this medication in total intact capsulectomy surgeries.
Objective
This study aimed to determine the influence of tranexamic acid on hematoma prevention in breast explantation surgery with intact total capsulectomy.
Methods
This retrospective study collected medical records data to determine the incidence of hematoma requiring surgical drainage in 280 patients sequentially operated on by the author from January 2022 to December 2023. The Research Ethics Committee of Plataforma Brasil evaluated this study under number 76656623.0.0000.5470.
All patients in the study underwent total intact capsulectomy with or without mastopexy; no subject had a new breast implant inserted. The only difference between the groups was the use of tranexamic acid or not. All patients had received breast implants for aesthetic reasons.
There is no reliable populational data regarding the annual explantation rate in Brazil. Sample calculation employed a population of 10,000 patients undergoing explantation annually with a margin of error of 5% and a confidence level of 90%, reaching a value of 264. Therefore, the sample consisted of 280 patients divided into two groups of 140 subjects.
From January to September 2022, 140 patients underwent total intact capsulectomy and several reconstruction types without tranexamic acid.
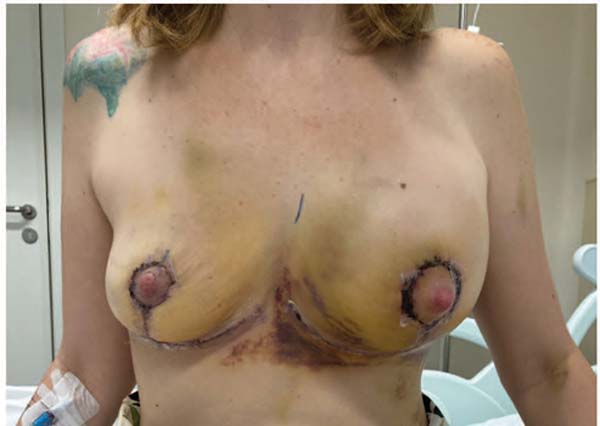
From October 2022 to December 2023, another 140 patients underwent total intact capsulectomy and several reconstruction types with the administration of 1 gram of tranexamic acid during anesthetic induction (►Fig. 1). In addition, each dissected breast received irrigation with 10 mL of a 10 mL solution containing 500 mg of tranexamic acid and 10 mL of saline, with a total volume of 20 mL.
All patients received a Blake 15 drain, kept until the 24-hour output was lower than 30 ml. Office follow-ups occur at 7, 30, and 90 days.
The study analyzed the quantitative and qualitative characteristics of the samples from both groups (►Tables 1 and 2).
| Receiving TA | Not receiving TA | Total | p value | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
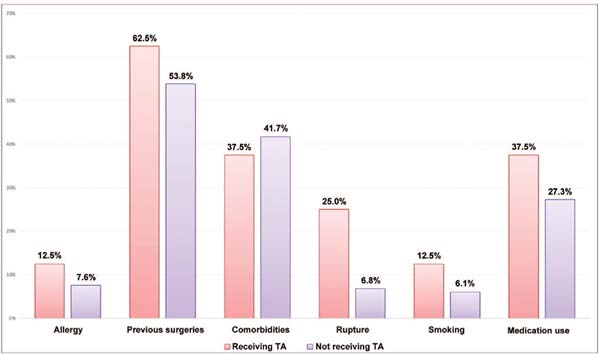
| Allergy | No | 125 | 89.3% | 129 | 92.1% | 254 | 90.7% | 0.410 |
| Yes | 15 | 10.7% | 11 | 7.9% | 26 | 9.3% | ||
| Previous surgeries | No | 60 | 42.9% | 64 | 45.7% | 124 | 44.3% | 0.630 |
| Yes | 80 | 57.1% | 76 | 54.3% | 156 | 55.7% | ||
| Comorbidities | No | 81 | 57.9% | 82 | 58.6% | 163 | 58.2% | 0.904 |
| Yes | 59 | 42.1% | 58 | 41.4% | 117 | 41.8% | ||
| Hematoma | No | 140 | 100% | 132 | 94.3% | 272 | 97.1% | 0.004 |
| Yes | 0 | 0% | 8 | 5.7% | 8 | 2.9% | ||
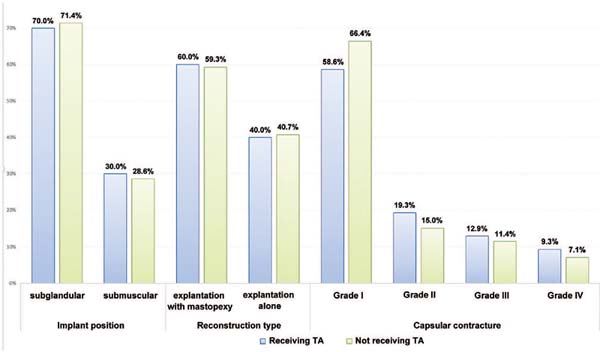
| Implant position | Subglandular | 98 | 70.0% | 100 | 71.4% | 198 | 70.7% | 0.793 |
| Submuscular | 42 | 30.0% | 40 | 28.6% | 82 | 29.3% | ||
| Rupture | No | 131 | 93.6% | 129 | 92.1% | 260 | 92.9% | 0.643 |
| Yes | 9 | 6.4% | 11 | 7.9% | 20 | 7.1% | ||
| Smoking | No | 133 | 95.0% | 131 | 93.6% | 264 | 94.3% | 0.607 |
| Yes | 7 | 5.0% | 9 | 6.4% | 16 | 5.7% | ||
| Reconstruction type | Explantation with mastopexy | 84 | 60.0% | 83 | 59.3% | 167 | 59.6% | 0.903 |
| Explantation alone | 56 | 40.0% | 57 | 40.7% | 113 | 40.4% | ||
| Medication use | No | 98 | 70.0% | 101 | 72.1% | 199 | 71.1% | 0.693 |
| Yes | 42 | 30.0% | 39 | 27.9% | 81 | 28.9% | ||
| Capsular contracture | Grade I | 82 | 58.6% | 93 | 66.4% | 175 | 62.5% | 0.583 |
| Grade II | 27 | 19.3% | 21 | 15.0% | 48 | 17.1% | ||
| Grade III | 18 | 12.9% | 16 | 11.4% | 34 | 12.1% | ||
| Grade IV | 13 | 9.3% | 10 | 7.1% | 23 | 8.2% | ||
| Mean | Median | SD | CV | Min | Max | N | CI | p value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Implant type | Receiving TA | 10.24 | 10.0 | 4.68 | 46% | 1.0 | 21.0 | 140 | 0.78 | 0.817 |
| Not receiving TA | 10.37 | 10.0 | 4.61 | 44% | 2.0 | 23.0 | 140 | 0.76 | ||
| Age | Receiving TA | 40.79 | 40 | 9.27 | 23% | 20 | 71 | 140 | 1.53 | 0.990 |
| Not receiving TA | 40.77 | 40 | 8.95 | 22% | 23 | 67 | 140 | 1.48 | ||
| BMI | Receiving TA | 23.77 | 23.4 | 3.63 | 15% | 16.9 | 36.4 | 140 | 0.60 | 0.686 |
| Not receiving TA | 23.58 | 22.6 | 3.86 | 16% | 16.9 | 38.6 | 140 | 0.64 | ||
| Implant size | Receiving TA | 291 | 295 | 63 | 22% | 120 | 500 | 140 | 11 | 0.648 |
| Not receiving TA | 295 | 290 | 70 | 24% | 150 | 500 | 140 | 12 | ||
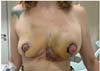
Moreover, this study recorded the incidence of hematoma requiring surgical drainage and performed statistical analysis to verify that the difference between the groups was not random. A new surgical treatment occurred in all hematoma cases (►Fig. 2).
Inclusion criteria:
Patients undergoing total intact capsulectomy
Patients over 18 years old
Patients with bilateral breast implants
Exclusion criteria:
Results
This study used parametric statistical tests after verifying the normality of the main outcome quantitative variables with the Shapiro-Wilks test (N ≥ 100). Parametric tests have more power to detect significance.
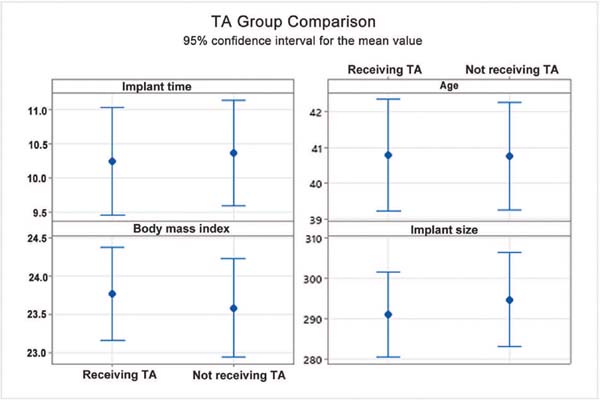
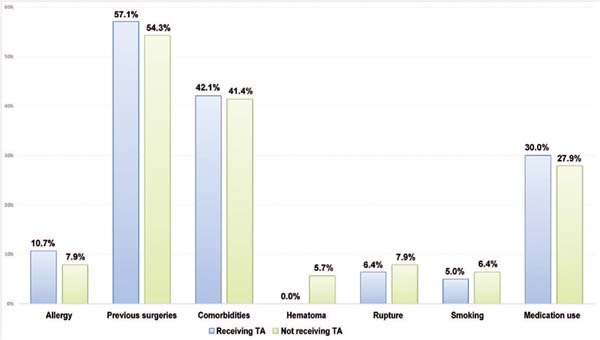
Excluding the incidence of hematoma, the groups with or without tranexamic acid were homogeneous since there was no statistically significant mean difference in quantitative (►Fig. 3) or qualitative variables (►Figs. 4 and 5).
Pearson’s chi-square test assessed whether there was an association between two qualitative variables, i.e., tranexamic acid and hematoma, and the p value was calculated (►Table 1).
The Student’s t-test assessed the association between two quantitative variables (►Table 2).
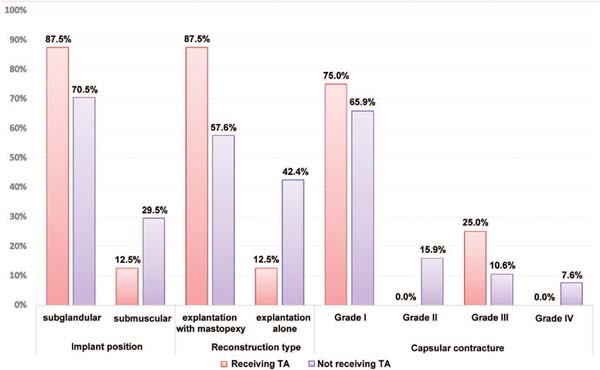
Hematomas occurred only in the group without tranexamic acid; as such, this group underwent an analysis of other factors (►Tables 3 and 4 and ►Fig. 6) using the Student’s t-test. The chi-square test evaluated qualitative factors.
| Hematoma | Mean | Median | SD | CV | Min | Max | N | CI | p value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Implant type | With hematoma | 10.38 | 11.0 | 3.42 | 33% | 5.0 | 15.0 | 8 | 2.37 | 0.998 |
| Without hematoma | 10.37 | 10.0 | 4.68 | 45% | 2.0 | 23.0 | 132 | 0.80 | ||
| Age | With hematoma | 41.00 | 41 | 6.37 | 16% | 30 | 51 | 8 | 4.41 | 0.941 |
| Without hematoma | 40.76 | 39 | 9.10 | 22% | 23 | 67 | 132 | 1.55 | ||
| BMI | With hematoma | 25.01 | 24.2 | 3.41 | 14% | 21.6 | 32.0 | 8 | 2.37 | 0.285 |
| Without hematoma | 23.50 | 22.6 | 3.88 | 17% | 16.9 | 38.6 | 132 | 0.66 | ||
| Implant size | With hematoma | 350 | 345 | 63 | 18% | 250 | 450 | 8 | 43 | 0.020 |
| Without hematoma | 291 | 283 | 69 | 24% | 150 | 500 | 132 | 12 | ||
| With hematoma | Without hematoma | Total | p value | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Allergy | No | 7 | 87.5% | 122 | 92.4% | 129 | 92.1% | 0.615 |
| Yes | 1 | 12.5% | 10 | 7.6% | 11 | 7.9% | ||
| Previous surgeries | No | 3 | 37.5% | 61 | 46.2% | 64 | 45.7% | 0.631 |
| Yes | 5 | 62.5% | 71 | 53.8% | 76 | 54.3% | ||
| Comorbidities | No | 5 | 62.5% | 77 | 58.3% | 82 | 58.6% | 0.816 |
| Yes | 3 | 37.5% | 55 | 41.7% | 58 | 41.4% | ||
| Implant position | Subglandular | 7 | 87.5% | 93 | 70.5% | 100 | 71.4% | 0.300 |
| Submuscular | 1 | 12.5% | 39 | 29.5% | 40 | 28.6% | ||
| Rupture | No | 6 | 75.0% | 123 | 93.2% | 129 | 92.1% | 0.063 |
| Yes | 2 | 25.0% | 9 | 6.8% | 11 | 7.9% | ||
| Smoking | No | 7 | 87.5% | 124 | 93.9% | 131 | 93.6% | 0.471 |
| Yes | 1 | 12.5% | 8 | 6.1% | 9 | 6.4% | ||
| Reconstruction type | Explantation with mastopexy | 7 | 87.5% | 76 | 57.6% | 83 | 59.3% | 0.094 |
| Explantation alone | 1 | 12.5% | 56 | 42.4% | 57 | 40.7% | ||
| Medication use | No | 5 | 62.5% | 96 | 72.7% | 101 | 72.1% | 0.531 |
| Yes | 3 | 37.5% | 36 | 27.3% | 39 | 27.9% | ||
| Capsular contracture | Grade I | 6 | 75.0% | 87 | 65.9% | 93 | 66.4% | 0.342 |
| Grade II | 0 | 0.0% | 21 | 15.9% | 21 | 15.0% | ||
| Grade III | 2 | 25.0% | 14 | 10.6% | 16 | 11.4% | ||
| Grade IV | 0 | 0.0% | 10 | 7.6% | 10 | 7.1% | ||
Side distribution of hematomas, which only occurred in the group without tranexamic acid used the two-proportion Z test (►Table 5).
| Hematoma | N | % | p value |
|---|---|---|---|
| Left | 3 | 37.5% | 0.317 |
| Right | 5 | 62.5% |
It was not possible to statistically verify the relationship between the side of breast implant rupture and the side of the hematoma. Among the 20 cases with breast implant rupture, only two had hematoma, and the rupture and the hematoma occurred on the left side.
Discussion
The search for reducing postoperative hematomas has been constant throughout the history of surgery. The advent of the electric scalpel, drains, and medications proved the efforts for hematoma reduction.
Tranexamic acid is the best candidate for the antifibrinolytic drug of choice, as it has as low cost, high hospital availability, safety in not increasing thromboembolic events, and few contraindications.
Studies indicate that 10μg/mL of tranexamic acid is required for 80% inhibition of plasminogen activation. This concentration translates into an intravenous dose of 10 mg/kg with adequate serum and tissue levels for 8 and 17 hours, respectively.10,15,16
The most common administration form includes a 10 mg/kg or 1 g bolus in anesthetic induction followed by a constant infusion of 1 mg/kg/hour or 1 g every 8 hours.10,16
In 1994, Oerli demonstrated that 1 g of tranexamic acid three times a day in patients undergoing mastectomy decreased drain output and hospital stay.17 In 2019, Knight showed that tranexamic acid in a single intravenous dose during surgery reduced the incidence of hematoma.18 Ausen et al., in 2015, reported that the topical use of tranexamic acid decreased drain output.19
The greatest contraindications to the intravenous administration of tranexamic acid include intracranial bleeding, a history of thromboembolic diseases, and allergy to the medication. High intravenous doses can cause complications such as seizures, especially in patients with a history of neurological diseases and renal dysfunction.16,20 Several studies have reported no increased risk of thromboembolic events with tranexamic acid.11,21
Topical tranexamic acid has reduced risks and is an alternative to intravenous use with a comparable effect in reducing hematomas, drain output, and the need for blood transfusion.22,23,24 Combined intravenous and topical use provides a hemostatic effect and reduces the risk of adverse effects.15 Although studies revealed that the plasma concentration with topical use is less than 10% of the intravenous level, the lowest effective topical concentration is unknown. In addition, it remains unclear whether the dose, the exposure time, or both influence its efficacy.11
In comparative studies, the efficacy of topical use is equal to or greater than intravenous use, with a 29% reduction in blood loss and a 45% reduction in the need for blood transfusion.10
The present study demonstrated that the groups receiving tranexamic acid or not were comparable in qualitative (comorbidities, allergies) and quantitative variables (implant size, body mass index). Since there was no statistically significant mean difference between the groups, they can be deemed homogeneous (►Figs. 3 4 5).
Group homogeneity is critical because biases cannot influence outcomes after introducing a new variable.
Group homogeneity is critical because biases cannot influence outcomes after introducing a new variable.
Our data showed that, in both groups, patients seeking explantation have an average age of 40 years old, are in good health, are not overweight, do not smoke or have allergies, and maintained a 300 mL subglandular breast implant for 10 years.
Regarding the surgical procedure, approximately 60% of the patients underwent explantation with mastopexy, while 40% underwent explantation only. Reasons for explantation included ruptured breast implants (7.1% of subjects) and grade III or IV capsular contracture (20.3% of patients). These data suggest that a significant portion of the patients opted for the explantation with no surgical indication.
There was a statistical significance between tranexamic acid and hematoma incidence (p value = 0.004). The group receiving tranexamic acid had no hematomas, while the group who did not receive it had an incidence of hematomas of 5.7%. The p value of 0.004 indicates a 0.4% probability that these findings were random; therefore, a strong association between these data shows that tranexamic acid prevents hematomas in breast explantation surgeries.
The hematoma rate in the group not receiving tranexamic acid ranged from 1 to 7% of breast surgeries.1
In addition, there was a statistical significance for the implant size. The average size was 350 mL for cases with hematoma versus 291 mL in the group without hematoma (p value = 0.020). This relationship between implant size and hematoma may be due to the larger dissection area in larger implants. It could be expected that major surgeries such as explantation with mastopexy or explantation of submuscular implants would have a higher incidence of hematoma. This argument would be justified due to the larger dissection area in major surgeries and the manipulation of the highly irrigated pectoral muscle. However, this study did not show statistically significant differences in the incidence of hematoma between the groups with submuscular or subglandular implants or between the groups undergoing explantation alone or explantation with mastopexy.
The difference in the incidence of hematoma between the breasts, with 62.5% occurring on the right side and 37.5% on the left side, had no significance, with a p value of 0.317.
A breast implant rupture results in loss of the original shape, increasing the dissection area for capsulectomy. However, there was no relationship between the breast side with the ruptured implant and the incidence of hematoma.
It is worth highlighting the limitations of a retrospective study, including the lack of randomization in patient allocation into groups and the absence of procedural standardization since some patients underwent explantation alone and others underwent explantation with mastopexy.
Conclusion
Topical and intravenous use of tranexamic acid reduces the incidence of hematoma after surgery involving intact total capsulectomy in patients with breast implants.
REFERENCES
1. Albanese R, Zingaretti N, Almesberger D, Parodi PC. Intravenous Tranexamic Acid in Implant-Based Breast Reconstruction Safely Reduces Hematoma without Thromboembolic Events. Plast Reconstr Surg 2022;149(01):139e–140e. United States
2. Miranda R. What is the impact of capsulectomy on systemic symptoms attributed to silicone breast implants? Rev Bras Cir Plást 2023;38(04):1–8
3. Brown S, Yao A, Taub PJ. Antifibrinolytic Agents in Plastic Surgery: Current Practices and Future Directions. Plast Reconstr Surg 2018;141(06):937e–949e
4. Afshari A, Nguyen L, Glassman GE, Perdikis G, Grotting JC, Higdon KK. Incidence and Preoperative Risk Factors for Major Complications After Capsulectomy: Analysis of 3048 Patients. Aesthet Surg J 2022;42(06):603–612
5. Locketz GD, Lozada KN, Bloom JD. Tranexamic Acid in Aesthetic Facial Plastic Surgery: A Systematic Review of Evidence, Applications, and Outcomes. Aesthet Surg J Open Forum 2020;2(03):ojaa029
6. Wolf Y, Skorochod R, Kwartin S, Shapira L Tranexamic Acid Irrigation in Liposuction: A Double-Blind, Half-Body, Randomized, Placebo-Controlled Trial. Aesthetic Plast Surg 2023
7. Al-Hashimi M, Kaur P, Charles W, Bhasta M, Nahai F, Khajuria A. A Systematic Review of the Efficacy and Safety of Tranexamic Acid in Facelift Surgery. Aesthet Surg J 2023;43(11):1211–1218
8. Liechti R, van de Wall BJM, Hug U, Fritsche E, Franchi A. Tranexamic Acid Use in Breast Surgery: A Systematic Review and Meta-Analysis. Plast Reconstr Surg 2023;151(05):949–957
9. Nayak LM, Linkov G. The Role of Tranexamic Acid in Plastic Surgery: Review and Technical Considerations. Plast Reconstr Surg 2018;142(03):423e. United States
10. Rohrich RJ, Cho MJ. The Role of Tranexamic Acid in Plastic Surgery: Review and Technical Considerations. Plast Reconstr Surg 2018; 141(02):507–515
11. Ausen K, Fossmark R, Spigset O, Pleym H. Safety and Efficacy of Local Tranexamic Acid for the Prevention of Surgical Bleeding in Soft-Tissue Surgery: A Review of the Literature and Recommendations for Plastic Surgery. Plast Reconstr Surg 2022; 149(03): 774–787
12. Adidharma W, Latack KR, Colohan SM, Morrison SD, Cederna PS. Breast Implant Illness: Are Social Media and the Internet Worrying Patients Sick? Plast Reconstr Surg 2020;145(01):225e–227e. United States
13. Bird GR, Niessen FB. The effect of explantation on systemic disease symptoms and quality of life in patients with breast implant illness: a prospective cohort study. Sci Rep 2022;12(01):21073
14. Metzinger SE, Homsy C, Chun MJ, Metzinger RC. Breast Implant Illness: Treatment Using Total Capsulectomy and Implant Removal. Eplasty 2022;22:e5
15. Abboud NM, Kapila AK, Abboud S, Yaacoub E, Abboud MH. The Combined Effect of Intravenous and Topical Tranexamic Acid in Liposuction: A Randomized Double-Blinded Controlled Trial. Aesthet Surg J Open Forum 2021;3(01):ojab002
16. Elena Scarafoni E. A Systematic Review of Tranexamic Acid in Plastic Surgery: What’s New? Plast Reconstr Surg Glob Open 2021;9(03):e3172
17. Oertli D, Laffer U, Haberthuer F, Kreuter U, Harder F. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg 1994;81(06):856–859
18. Knight H, Banks J, Muchmore J, Ives C, Green M. Examining the use of intraoperative tranexamic acid in oncoplastic breast surgery. Breast J 2019;25(05):1047–1049
19. Ausen K, Fossmark R, Spigset 0, Pleym H. Randomized clinical trial of topical tranexamic acid after reduction mammoplasty. Br J Surg 2015;102(11):1348–1353
20. Hoyos AE, Duran H, Cardenas-Camarena L, et al. Use of Tranexamic Acid in Liposculpture: A Double-Blind, Multicenter, Randomized Clinical Trial. Plast Reconstr Surg 2022;150(03):569–577
21. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res 2009;123(05):687–696
22. Wang S, Yang J, Lin L Local Application of Tranexamic Acid in Plastic Surgery Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Aesthetic Plast Surg 2023;47 (04):1633–1643
23. Safran T, Vorstenbosch J, Viezel-Mathieu A, Davison P, Dionisopoulos T. Topical Tranexamic Acid in Breast Reconstruction: A Double-Blind Randomized Controlled Trial. Plast Reconstr Surg 2023;152(04):699–706
24. Wheeler DR, Bucci F, Vaccari S, di Giuli R, Vinci V, Klinger M. Topical Tranexamic Acid: Risks, Benefits and Novel Complications in Aesthetic Plastic Surgery. Aesthetic Plast Surg 2023;47(06): 2880–2888
1. Private practice, plastic surgery, São Paulo,
SP, Brazil
Address for correspondence Ricardo Eustachio de Miranda, Rua Bandeira Paulista, 530, sala 43, São Paulo, SP, Brazil CEP 04532-001 (e-mail: ricardomiranda@hotmail.com; drmiranda78@gmail.com).
Article received: March 02, 2024.
Article accepted: September 29, 2024.
Conflict of Interests
The author has no conflict of interests to declare.













 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter