

Review Article - Year 2024 - Volume 39 -
The Risks of Polymethyl Methacrylate: An Integrative Review of 587 Complication Reports
Os riscos do polimetilmetacrilato: Revisão integrativa de 587 casos de complicações
ABSTRACT
Introduction The demand for minimally-invasive esthetic procedures has boosted the use of polymethyl methacrylate (PMMA) as a dermal filler, which was initially approved for human immunodeficiency virus (HIV)-related lipodystrophies. Improper PMMA use can lead to severe complications, including inflammation, material migration, infections, and systemic repercussions. The present review investigated PMMA-related risks and complications reported in the literature over the past 20 years.
Materials and Methods We conducted an integrative review of descriptive studies on PMMA complications published from 2004 to 2024 and retrieved from PubMed, Scientific Electronic Library Online (SciELO), and Scopus. We analyzed 38 studies, encompassing 587 cases, and excluded articles published outside the specified period and those not meeting the inclusion criteria.
Results We identified 587 cases of PMMA-related complications, 64% of them occurring in Brazil. The most common complication site was the malar region followed by the nasolabial fold, the gluteal area, and the lips. The complications ranged from immediate to delayed, and from mild to severe. The most frequent complications included erythema (26%), pruritus (16%), edema (11%), granulomas (21%), nodules (14%), and skin changes (8%). Severe cases included renal failure, hypercalcemia, septic shock, infections, and tissue necrosis. The volume injected ranged from 1.9 to 900mL. The treatment predominantly involved steroids, antibiotics, and surgical interventions, including excisions, abscess drainage, and debridement.
Conclusion The use of PMMA fillers may lead to severe complications, often unrelated to the correction of HIV-related lipodystrophies; PMMA presents significant risks, including systemic complications, requiring surgical removal.
Keywords: case reports; dermal fillers; polymethyl methacrylate; review; risk
RESUMO
Introdução A demanda por procedimentos estéticos minimamente invasivos impulsionou o uso do polimetilmetacrilato (PMMA) como preenchimento dérmico, que a princípio foi aprovado para lipodistrofias relacionadas ao vírus da imunodeficiência humana (HIV - human immunodeficiency virus, em inglês). Seu uso inadequado causa graves complicações, como inflamações, migração do material, infecções e repercussões sistêmicas. Nesta revisão, objetivamos realizar uma investigação sobre esses riscos e complicações relatadas na literatura nos últimos 20 anos.
Materiais e Métodos Realizamos uma revisão integrativa de estudos descritivos sobre complicações do PMMA publicados entre 2004 e 2024, utilizando as bases de dados PubMed, Scientific Electronic Library Online (SciELO) e Scopus. Foram analisados 38 estudos, totalizando 587 casos, e foram excluídos artigos publicados fora do período analisado ou que não atendiam aos critérios de inclusão.
Resultados Encontramos 587 casos de complicações associadas ao PMMA, sendo que 64% deles ocorreram no Brasil. O sítio mais frequente foi a região malar, seguido do sulco nasogeniano, da região glútea, e dos lábios. As complicações variaram entre imediatas e tardias, e de repercussões simples a mais severas. As mais frequentes foram: eritema (26%), prurido (16%), edema (11%), granulomas (21%), nodulações (14%) e alterações cutâneas (8%), incluindo casos graves de insuficiência renal, hipercalcemia, choque séptico, infecções e necrose de tecido. A quantidade injetada de material variou de 1,9 a 900 mL, e, no que tange ao tratamento, houve predominância de esteroides, antibióticos e necessidade de abordagem cirúrgica, com realização de excisões, drenagem de abscesso e desbridamento.
Conclusão Preenchimentos com PMMA estão associados a complicações graves, e seu uso muitas vezes não está relacionado à correção de lipodistrofias por HIV. O material tem riscos significativos, incluindo complicações sistêmicas, com a necessidade de remoção cirúrgica.
Palavras-chave: polimetilmetacrilato; preenchedores dérmicos; relatos de casos; revisão; risco
Introduction
The demand for minimally-invasive aesthetic procedures promising efficient, satisfactory results and a short recovery time has increased in recent years.1 Cosmetic procedures, such as dermal fillers and facial and body volumizers, have emerged as popular alternatives to conventional plastic surgery, promising quick recovery and giving the false idea of fewer risks, meeting patients’ expectations.2
In this context, polymethyl methacrylate (PMMA) is a widely used filler. It is a non-absorbable plastic component in spheres inserted into a vehicle to enable injectable application.3 The initial approval of PMMA for outpatient use in Brazil occurred in 2009 by the Ministry of Health for the treatment of lipodystrophies secondary to the adverse effects of antiretrovirals for HIV treatment.4,5 However, over the years, the indiscriminate and inappropriate use of PMMA has resulted in numerous immediate and delayed complications.3
Immediate PMMA complications include inflammatory reactions, pain, and infections shortly after the application. Such complications can range from minor adverse reactions, such as erythema and edema, to serious adverse effects, including tissue necrosis, ocular involvement, severe infections, and renal failure due to hypercalcemia.5-7 Acute complications can persist and evolve into granulomatous formations, recurrent infections, autoimmune diseases, and chronic renal failure. In addition, material migration to undesirable areas can result in local asymmetries and deformities many years after PMMA application.8
As a non-absorbable material, the interaction of PMMA with the adjacent tissue hinders its isolated removal. The irreversible nature of PMMA application is a major limitation.3,5,8 In the face of complications such as granulomas, chronic inflammatory reactions, fibrosis, and infections with bacterial biofilm formation, the only therapeutic alternative is surgical removal of the PMMA together with the adjacent healthy tissue, resulting in permanent sequelae.5,8
Inappropriate application techniques, questionable indications, inadequate PMMA volumes for the target treatment area, and poor selection of the quality of the materials can intensify these reactions. Studies6 show that qualified plastic surgeons or dermatologists who understand the anatomy of the area to be treated, master the correct technique, and know how to treat potential complications should perform the procedure; however, these recommendations are often not considered.
As such, a broad investigation in the medical literature is required to compile the available scientific evidence on PMMA, determine the potential immediate and delayed complications resulting from its use, increase the understanding of PMMA safety for esthetic purposes, and question the approval of its use by the competent bodies.
Objective
In the present study, we conducted an integrative review of immediate and delayed complications resulting from PMMA reported in the literature over the last 20 years.
Materials and Methods
The present is an integrative review of the literature covering case reports and case series on immediate and delayed PMMA-related complications. We searched for articles published from 2004 to 2024 in the PubMed, Scientific Electronic Library Online (SciELO), and Scopus databases using the descriptors polymethyl methacrylate, dermal fillers, and complications.
We excluded from the review studies published outside the stipulated period, experimental studies, and studies not conducted in humans. Two independent reviewers fully read the studies meeting the inclusion criteria and, in case of conflict, a third researcher solved the discrepancy.
We extracted the following data from the articles: journal’s name, author, year, country of origin, type of study (case report or case series), casuistry, age, professional performing the procedure (physician or non-physician), injected volume, anatomical site treated, post-PMMA injection signs and symptoms, immediate complications, delayed complications, complication treatment, and whether the filler treated HIV-related lipodystrophy or was used for esthetic purposes.
Results
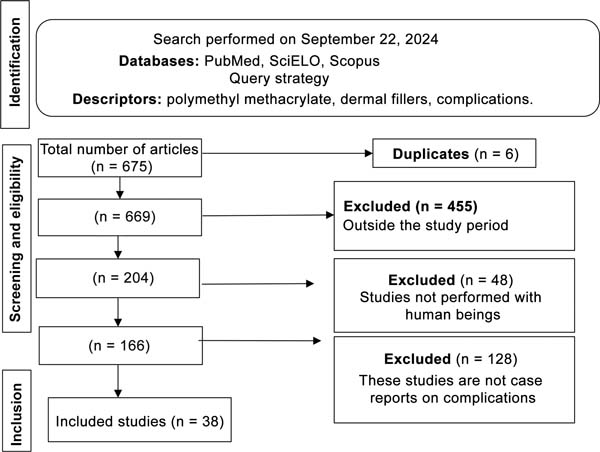
Overall, 38 studies met the inclusion criteria for this review, totaling 587 cases (►Fig. 1): 17 articles were case series and 21 were individual case reports (►Table 1);9 39% of the articles (15/38) reported cases occurring in Brazil; the oldest publication5 dates from 2008 and the most recent one,9 from 2024 (►Table 2).
| Journal | Author | Year | Casuistry | Reference |
|---|---|---|---|---|
| Revista Brasileira de Cirurgia Plástica | Goldman et al. | 2024 | 209 | 9 |
| Annals of Plastic Surgery | Durkin et al. | 2023 | 3 | 10 |
| Journal of the American Academy of Dermatology | Vengalil et al. | 2023 | 1 | 11 |
| Cytopathology | Saoud et al. | 2023 | 1 | 12 |
| Ophthalmic Plastic and Reconstructive Surgery | Parikh et al. | 2023 | 1 | 13 |
| Journal of Cutaneous and Aesthetic Surgery | Sivam et al. | 2023 | 1 | 14 |
| Journal of Drugs in Dermatology | Goldman et al. | 2021 | 27 | 15 |
| SKIN The Journal of Cutaneous Medicine | Dhaliwal et al. | 2021 | 1 | 16 |
| European Review for Medical and Pharmacological Sciences | Freire de Carvalho et al. | 2021 | 1 | 17 |
| Annals of Medicine and Surgery | Alimoradi et al. | 2021 | 1 | 18 |
| Brazilian Journal of Nephrology | Manfro et al. | 2021 | 2 | 7 |
| Revista Brasileira de Cirurgia Plástica | Kurimori et al. | 2019 | 1 | 3 |
| Dermatologic Therapy | Goldman and Wollina | 2019 | 2 | 19 |
| Dermatologic Surgery | Ibrahim and Dover | 2018 | 1 | 20 |
| Georgian Medical News | Goldman et al. | 2018 | 2 | 21 |
| Open Access Macedonian Journal of Medical Sciences | Goldman and Wollina | 2018 | 81 | 22 |
| Contact Dermatitis | Shah et al. | 2017 | 1 | 23 |
| Revista Brasileira de Cirurgia Plástica | Souza et al. | 2016 | 1 | 24 |
| Diagnostic Pathology | Cannata-Ortiz et al. | 2016 | 1 | 25 |
| Aesthetic Surgery Journal | Limongi et al. | 2016 | 11 | 26 |
| Plastic Reconstructive Surgery - Global Open | Purnell et al. | 2016 | 1 | 27 |
| Journal of Cosmetic & Laser Therapy | Friedmann et al. | 2016 | 4 | 28 |
| Medicina Intensiva | Boattini et al. | 2015 | 1 | 29 |
| Calcified Tissue International | Hindi et al. | 2015 | 1 | 30 |
| Skin Research and Technology | Cinotti et al. | 2015 | 1 | 31 |
| Archives of Endocrinology and Metabolism | Rados and Furlanetto | 2015 | 1 | 32 |
| Clinical Cases in Mineral and Bone Metabolism | Negri et al. | 2014 | 4 | 33 |
| Journal of the American Geriatrics Society | Costa et al. | 2014 | 1 | 34 |
| Aesthetic Plastic Surgery | Park et al. | 2012 | 13 | 35 |
| Journal of Plastic, Reconstructive & Aesthetic Surgery | Park et al. | 2012 | 15 | 36 |
| The Canadian Journal of Plastic Surgery | Solomon et al. | 2012 | 153 | 37 |
| Journal of Hand Surgery | Al-Qattan | 2011 | 3 | 38 |
| Journal of Plastic, Reconstructive & Aesthetic Surgery | Santana et al. | 2010 | 1 | 39 |
| Aesthetic Plastic Surgery | de Figueiredo et al. | 2010 | 1 | 40 |
| Kosmetische Medizin | Wollina | 2009 | 1 | 41 |
| Plastic and Reconstructive Surgery | Salles et al. | 2008 | 32 | 5 |
| Dermatology | Wolfram et al. | 2006 | 4 | 42 |
| Clinical and Experimental Dermatology | Sidwell et al. | 2006 | 1 | 43 |
| Total | 587 | |||
| Author | Year | Number of cases | Reference |
|---|---|---|---|
| Goldman et al. | 2024 | 209 | 9 |
| Goldman et al. | 2021 | 27 | 15 |
| Freire de Carvalho | 2021 | 1 | 17 |
| Manfro et al. | 2021 | 2 | 7 |
| Kurimori et al. | 2019 | 1 | 3 |
| Goldman and Wollina | 2019 | 2 | 19 |
| Goldman et al. | 2018 | 2 | 21 |
| Goldman and Wollina | 2018 | 81 | 22 |
| Souza et al. | 2016 | 1 | 24 |
| Limongi et al. | 2016 | 11 | 26 |
| Rados and Furlanetto | 2015 | 1 | 32 |
| Costa et al. | 2014 | 1 | 34 |
| Santana et al. | 2010 | 1 | 39 |
| de Figueiredo et al. | 2010 | 1 | 40 |
| Salles et al. | 2008 | 32 | 5 |
| Total | 373 | ||
Anatomical Regions and Injected Volume
The most frequent filler administration site was the malar region, followed by the nasolabial fold, the gluteal region, the lips, the nasal region, the frontal region, the face, the eyelid region, the zygomatic region, the upper limbs, the chin, the jaw, the chest, the trunk, the lower limbs, the and temporal, auricular, and penile regions (►Table 3). Only 2 (5%) studies reported using fillers to reconstruct antiretroviral-related lipodystrophy.30,33 Most studies (89.4%) did not report the volume injected during the procedure; however, quantities ranged from 1.9 mL for the eyelid region in one patient,10 4 mL for facial treatment,17 and 2 cases of gluteal filling injecting 400 mL and 900 mL of PMMA.3,27
| Anatomical region | Number of studies (percentage) | Reference |
|---|---|---|
| Malar | 12 (0.13) | 5,9,11,13,14,19,20,28,35,39,42,43 |
| Nasolabial fold | 11 (0.12) | 9,20,24,28,31,35,37,39-42 |
| Gluteus | 10 (0.11) | 3,5,7,18,21,27,29,30,32,33 |
| Nose | 8 (0.09) | 5,9,15,19,23,28,37,40 |
| Frontal | 8 (0.09) | 5,9,11,12,28,35,37,42 |
| Lip | 7 (0.08) | 9,13,19,28,35,37,42 |
| Face (undetermined specific location) | 7 (0.08) | 16,19,22,26,35,39,43 |
| Ocular | 4 (0.04) | 9,10,35,42 |
| Zygomatic | 4 (0.04) | 9,17,42,43 |
| Upper limbs | 4 (0.04) | 7,19,21,38 |
| Mentum | 4 (0.04) | 9,13,28,42 |
| Mandibular | 2 (0.02) | 9,19 |
| Trunk | 2 (0.02) | 5,22 |
| Lower limbs | 2 (0.02) | 25,33 |
| Thorax | 1 (0.01) | 21 |
| Temporal | 1 (0.01) | 9 |
| Auricular | 1 (0.01) | 9 |
| Penis | 1 (0.01) | 5 |
Professional Performing the Procedure
In total, 6 out of 38 studies reported the professionals performing the procedure. In 27 cases, physicians performed the procedure, including 16 certified plastic surgeons, 9 dermatologists, and 2 urologists. Six filler procedures were performed by non-physicians, including nurses, estheticians, and dentists.3,5,11,13,28,31
Complications
The reported complications were divided into immediate (up to 30 days after PMMA injection) and delayed (after 30 days). Ten different immediate complications occurred; erythema was the most frequent (26%), followed by pruritus (16%), edema (11%), acute renal failure (11%), nodules (11%), and other less frequent conditions (►Table 4).
| Immediate | ||
|---|---|---|
| Type | Number of studies (percentage) | Reference |
| Erythema | 5 (0.26) | 13,23,37,38,42 |
| Pruritus | 3 (0.16) | 23,37,40 |
| Edema | 2 (0.11) | 23,38 |
| Acute kidney failure | 2 (0.11) | 3,29 |
| Nodule formation | 2 (0.11) | 35,42 |
| Septic shock | 1 (0.05) | 29 |
| Hepatic dysfunction | 1 (0.05) | 29 |
| Ecchymosis | 1 (0.05) | 37 |
| Respiratory failure | 1 (0.05) | 29 |
| Seropurulent secretion | 1 (0.05) | 40 |
| Delayed | ||
| Type | Number of studies (percentage) | Reference |
| Granuloma | 17 (0.21) | 5,9,10,12-14,16,18,21,22,26,30,33,38,39,41,42 |
| Nodule formation | 11 (0.14) | 9,11,19,24,26,28,31,34,35,37,43 |
| Skin abnormalities | 6 (0.08) | 25-28,38,42 |
| Inflammatory signs | 5 (0.06) | 5,9,32,36,38 |
| Edema | 5 (0.06) | 9,13,20,25,26 |
| Infection | 4 (0.05) | 9,17,28,42 |
| Necrosis | 3 (0.04) | 5,9,40 |
| Facial irregularity | 3 (0.04) | 26,36,42 |
| Hypercalcemia | 2 (0.03) | 18,33 |
| Ocular involvement | 2 (0.03) | 13,17 |
| Chronic kidney failure | 2 (0.03) | 7,21 |
| Reduced renal function | 1 (0.01) | 33 |
| Neovascularization | 1 (0.01) | 9 |
| Pigmentation | 1 (0.01) | 9 |
| Lacrimation | 1 (0.01) | 9 |
| Fistula | 1 (0.01) | 9 |
| Allergic reaction | 1 (0.01) | 13 |
| Psoriasis | 1 (0.01) | 17 |
| Nephrocalcinosis | 1 (0.01) | 18 |
| Pulmonary granulomatous lymphadenitis | 1 (0.01) | 18 |
| Pericarditis | 1 (0.01) | 21 |
| Sarcoidosis-like syndrome | 1 (0.01) | 21 |
| Erythema | 1 (0.01) | 26 |
| Tachycardia | 1 (0.01) | 27 |
| Hyperkalemia | 1 (0.01) | 32 |
| Nephritis | 1 (0.01) | 33 |
| Pruritus | 1 (0.01) | 33 |
| Hypertension | 1 (0.01) | 33 |
| Rigidity | 1 (0.01) | 36 |
| Seroma | 1 (0.01) | 38 |
| Pulmonary sarcoidosis | 1 (0.01) | 43 |
Thirty different delayed complications were reported. The most common delayed complication was granuloma (21%), followed by nonspecific nodules (14%), skin abnormalities (8%), inflammatory reactions (6%), edema (6%), infection (5%), necrosis (4%), facial irregularities (4%), hypercalcemia (3%), ocular involvement (3%), and chronic renal failure (3%), among others (►Table 4).
Treatment
The three most frequent treatments included steroids,3,7,9-11,13,14,17,18,21,24,26-28,30,32,33,35,36,38,39,41-43 antibiotics3,11,20,25,29,40,42,43 and surgeries,3,7,9,10,12,13,17,23 24,26-28,35,36,38-40,42 including 75 cases of excision. Other reported therapeutic approaches included laser,9,15,19,21,22 fluorouracil,9,14,28 ozone,9 xylitol,9 allopurinol,9 methotrexate,17 vitamin D,17 hydroxychloroquine,17 colchicine,21 hemodyalisis,7,29 vasopressor support,29 orotracheal intubation with ventilatory support,29 calcitonin,32,33 saline infusion,32 bisphosphonates,30,32,33 cyclophosphamide,33 ketoconazole,33 and hyaluronic acid,36,41 selected according to each case.
Discussion
Some literature reviews5 highlight the large number of complications resulting from PMMA fillers. In the present study, we updated the evidence regarding the number of complication reports resulting from PMMA use in the last 20 years. We identified a significant prevalence of cases occurring in Brazil, representing more than 50% of the total articles in the current review. Regulatory factors related to the history of Brazilian public health policy in the treatment of HIV/AIDS patients may explain these findings.
In 1996, the emergence of highly-active antiretroviral treatment improved the health condition of HIV/AIDS patients but also resulted in stigmatizing anatomical changes.44 To address this issue, the Brazilian National Health Regulatory Agency (Agência Nacional de Vigilância Sanitária, ANVISA, in Portuguese) authorized the use of PMMA fillers for reparative purposes in this population.45
The evolution of antiretroviral drugs decreased the number of patients with stigmatizing lipodystrophies. However, it is intriguing to note that the number of complications related to the esthetic use of PMMA has increased. In the current review, only two cases of complications resulted from PMMA use for reparative purposes in the treatment of HIV/AIDS patients.30,33
Our findings show that the current use of PMMA exceeds its established safe limits and formal indications determined by regulatory agencies. For instance, we detected case reports of gluteal fillers with volumes of up to 900 mL of PMMA,3 while the Brazilian consensual recommendation published by Souza et al.46 in 2017 is not to exceed the maximum volume of 100 mL in this region.
As such, it is important to highlight some points regarding the authorization of PMMA use for esthetic purposes. The lack of appropriate supervision results in inappropriate practices in PMMA use, such as procedures performed by non-medical professionals or untrained physicians, and in sites with inadequate infrastructure.3,5,11,13,28,31 Most articles, except for 6, did not identify the filler prescriber. Out of 34 cases, only 16 were performed by professionals certified for PMMA application, potentially increasing the procedural risk.5
From 2004 to 2024, the number of PMMA-related complications in Brazil was significantly higher compared with the rest of the world, totaling 373 cases. Although PMMA use for esthetic purposes has been questioned by the Brazilian Society of Plastic Surgery (Sociedade Brasileira de Cirurgia Plástica, SBCP, in Portuguese)47 this material remains widely employed as a filler in Brazil. In contrast, other countries often use different reversible and absorbable fillers, such as hyaluronic acid, autologous fat grafts, poly-L-lactic acid, and collagen biostimulators.48
The reports included more cases of delayed complications compared with immediate complications. The most frequent delayed complication was granulomatous formations (21%). It is worth noting the potential severity of the complications related to PMMA fillers, such as septic shock and chronic renal failure.7,21,29 In addition, the long-term safety of PMMA is uncertain, since there are reports12 of foreign body granulomas in regions distant from the PMMA injection site. Therefore, to potentially reduce the incidence of adverse effects, stricter monitoring of the distribution, sale, prescription, and procedural sites is required, in addition to raising public awareness of PMMA-related risks.6
In Brazil, ANVISA authorizes PMMA application in two situations: correction of lipodystrophy resulting from antiretroviral medications and volumetric correction of the face and body. Although there is no contraindication to PMMA use in the buttocks, it should not occur for volume increase. Only properly-qualified physicians must perform the application and decide, based on a detailed evaluation, the dosage and number of injections appropriate for each patient, considering the particularities and needs of the subject.49
It is worth noting that ANVISA classifies PMMA as an implantable medical product of risk class IV. This classification presupposes a high level of regulatory control due to the potential risks of complications, such as inflammatory reactions, infections, material migration, and tissue necrosis, especially because PMMA is a permanent material that is not resorbable by the body, highlighting the irreversibility of this filling method.49
Safer alternatives to PMMA include hyaluronic acid and autologous fat. Hyaluronic acid presents greater biocompatibility, a lower risk of complications, and the possibility of reversal by applying the enzyme hyaluronidase. Autologous fat is another viable and safe option with a lower complication risk.50
The limitations of the current review include the uncertainty as to whether the higher complication incidence in Brazil results from a higher frequency of PMMA procedures or an actual higher incidence of complications. This is a significant limitation, since we do not have absolute data on the total number of procedures performed in Brazil and worldwide. Another limitation is that the review is based only on cases of complications documented in the literature, which may not reflect reality. The number of complications is likely much higher than reported, since complications not published in scientific articles could not be included.
Conclusion
Polymethyl methacrylate fillers are associated with serious immediate and delayed complications. Most complications are not related to their use for the correction of lipodystrophies in patients with HIV/AIDS. Most (64%) complications described in the medical literature over the last 20 years occurred in Brazil. Polymethyl methacrylate is non-absorbable, and multiple invasive surgical procedures may be required to treat the sequelae resulting from its use as a permanent filler. There are alternatives to PMMA that are reversible and present lower risks to the health of patients.
REFERENCES
1. Richards BG, Schleicher WF, D’Souza GF, Isakov R, Zins JE. The Role of Injectables in Aesthetic Surgery: Financial Implications. Aesthet Surg J 2017;37(09):1039-1043. Doi: 10.1093/asj/sjx136
2. de Maio M. The minimal approach: an innovation in facial cosmetic procedures. Aesthetic Plast Surg 2004;28(05): 295-300. Doi: 10.1007/s00266-004-0037-1
3. Kurimori KT, Mendes M, Milcheski DA, Monteiro AA Junior, Gemperli R. Severe complication due to inappropriate use of polymethylmethacrylate: a case report and current status in Brazil. Rev Bras Cir Plást 2019;34(01):156-162
4. Martins WH, Pessôa KVO, Martins MA, Silva MH, Pereira Filho GV, Abreu LC. Facial filling with polymethylmethacrylate in patients with acquired immunodeficiency syndrome. Rev Bras Cir Plást 2016;31(02):216-228
5. Salles AG, Lotierzo PH, Gemperli R, et al. Complications after polymethylmethacrylate injections: report of 32 cases. Plast Reconstr Surg 2008;121(05):1811-1820. Doi: 10.1097/PRS.0b013e31816b1385
6. Martins EL, Kock PA, Fedatto PF. Polimetilmetacrilato (PMMA) na prática clínica: Revisão integrativa sobre abordagens estéticas, complicações e aspectos regulatórios. Research. Soc Dev 2024;13 (06):e9013646046 Available from https://rsdjournal.org/index.-php/rsd/article/view/46046[Internet]
7. Manfro AG, Lutzky M, Dora JM, Kalil MAS, Manfro RC. Case reports of hypercalcemia and chronic renal disease due to cosmetic injections of polymethylmethacrylate (PMMA). J Bras Nefrol 2021; 43(02): 288-292https://www.scielo.br/pdf/jbn/2020nahead/2175-8239-jbn-2020-0044.pdf
8. Cordeiro G, Salotti Ldos RAmbrósio LHC, De Oliveira Júnior MR, Maciel LTR, Coêlho MDG. Reações do PMMA nos procedimentos estéticos faciais. BrazilianJournal of Health Review. 2023;6(06): 27030-27042
9. Goldman A, Marinowic DR, Luz PM. Complicações relacionadas ao uso de polimetilmetacrilato na face: Análise de 209 casos. Rev Bras Cir Plást 2024;39(02):e0900
10. Durkin AJ, Catena D, Woltjen N, et al. Surgical Management of Polymethylmethacrylate-Collagen Gel Complications in the Lower Eyelid: A Case Series. Ann Plast Surg 2023;90(01):12-18. Doi: 10.1097/SAP.0000000000003364
11. Vengalil N, Council LM, Michalski BM. Foreign body granulomas to polymethylmethacrylate soft tissue filler following COVID-19 infection. JAAD Case Rep 2023;41:1-3. Doi: 10.1016/j.jdcr.2023.08.037
12. Saoud C, Lossos C, Ali SZ. Polymethylmethacrylate-induced foreign body reaction presenting as bilateral parotid lesions: A case report of dermal filler adverse reaction diagnosed on fine needle aspiration. Cytopathology 2023;34(04):385-387. Doi: 10.1111/cyt.13233
13. Parikh AO, Conger JR, Sibug Saber ME, Samimi D, Burnstine MA. Multiple cases of facial disfigurement from filler use and one injector. Ophthalmic Plast Reconstr Surg 2023;39(04):366-369. Doi: 10.1097/IOP.0000000000002323
14. Sivam S, Mackay C, Humphrey C, Kriet JD. Giant PMMA foreign body granulomas with imaging. J Cutan Aesthet Surg 2023;16 (03):256-258. Doi: 10.4103/JCAS.JCAS_194_20
15. Goldman A, Wollina U, Machado D, Marionwic D. Laser in the Treatment of Granulomas on the Nose Produced by Polymethylmethacrylate: A Case Series. J Drugs Dermatol 2021;20(11): 1161-1166. Doi: 10.36849/jdd.6550
16. Dhaliwal P, Ibad S, Losak D, Cockerell C. A Case of Granulomatous Hypersensitivity Reactions to a Dermal Filler Precipitated by PD-1 Checkpoint Inhibitor Therapy. Skin (Milwood, NY) 2021;5(01): 41-45. Doi: 10.25251/skin.5.1.10
17. Freire de Carvalho J. Polyautoimmunity (Psoriasis, Sjogren’s syndrome, and autoimmune uveitis) following polymethylmethacrylate injection. Eur Rev Med Pharmacol Sci 2021;25(06): 2478-2480. Doi: 10.26355/eurrev_202103_25410
18. Alimoradi M, Chahal A, El-Rassi E, Daher K, Sakr G. Synthol systemic complications: Hypercalcemia and pulmonary granulomatosis. A case report. Ann Med Surg (Lond) 2021; 69:102771. Doi: 10.1016/j.amsu.2021.102771
19. Goldman A, Wollina U. Polymethylmethacrylate-induced nodules of the lips: Clinical presentation and management by intralesional neodymium:YAG laser therapy. Dermatol Ther 2019;32(01): e12755. Doi: 10.1111/dth.12755
20. Ibrahim O, Dover JS. Delayed-onset nodules after polymethyl methacrylate injections. Dermatol Surg 2018;44(09):1236-1238. Doi: 10.1097/DSS.0000000000001405
21. Goldman A, Staub H, Wollina U. HYPERCALCEMIA DUE TO POLY- METHYLMETHACRYLATE INJECTIONS? (LITERATURE REVIEW AND CASE REPORTS). Georgian Med News 2018;9(282):17-20
22. Goldman A, Wollina U. Intralesional Neodymium YAG laser to Treat Complications of Polymethylmethacrylate. Open Access Maced J Med Sci 2018;6(09):1636-1641. Doi: 10.3889/oamjms.2018.348
23. Shah V, Chaubal TV, Bapat RA, Shetty D. Allergic contact dermatitis caused by polymethylmethacrylate following intradermal filler injection. Contact Dermatitis 2017;77(06):407-408. Doi: 10.1111/cod.12779
24. Souza RN, Mendoça SG, Alencar EC, França ALA, Araújo ÊG, Leite LAS. Complicação tardia de preenchimento cutâneo após facelift: relato de caso. Rev Bras Cir Plást 2016;31(02):269-272
25. Cannata-Ortiz P, Gracia C, Aouad Y, et al. Small vessel microembolization and acute glomerulonephritis following infection of aesthetic filler implants. Diagn Pathol 2016;11:2. Doi: 10.1186/s13000-016-0453-y
26. Limongi RM, Tao J, Borba A, et al. Complications and Management of Polymethylmethacrylate (PMMA) Injections to the Midface. Aesthet Surg J 2016;36(02):132-135. Doi: 10.1093/asj/sjv195
27. Purnell CA, Klosowiak JL, Cheesborough JE, Park E, Bandy A, Dumanian GA. Resolution of Cosmetic Buttock Injection-induced Inflammatory Reaction and Heart Failure after Excision of Filler Material. Plast Reconstr Surg Glob Open 2016;4(10):e1079. Doi: 10.1097/GOX.0000000000001079
28. Friedmann DP, Kurian A, Fitzpatrick RE. Delayed granulomatous reactions to facial cosmetic injections of polymethylmethacrylate microspheres and liquid injectable silicone: A case series. J Cosmet Laser Ther 2016;18(03):170-173. Doi: 10.3109/14764172.2015.1114642
29. Boattini M, Francisco AR, Cavaco R, Rodrigues J, Bento L. Shock following subcutaneous injections of polymethylmethacrylate. Med Intensiva 2015;39(04):256-257. Doi: 10.1016/j.medin.2014.06.011
30. Hindi SM, Wang Y, Jones KD, et al. A Case of Hypercalcemia and Overexpression of CYP27B1 in Skeletal Muscle Lesions in a Patient with HIV Infection After Cosmetic Injections with Polymethylmethacrylate (PMMA) for Wasting. Calcif Tissue Int 2015;97(06): 634-639. Doi: 10.1007/s00223-015-0048-8
31. Cinotti E, Perrot JL, Labeille B, et al. Identification of a soft tissue filler by ex vivo confocal microscopy and Raman spectroscopy in a case of adverse reaction to the filler. Skin Res Technol 2015;21 (01):114-118. Doi: 10.1111/srt.12166
32. Rados DV, Furlanetto TW. An unexpected cause of severe and refractory PTH-independent hypercalcemia: case report and literature review. Arch Endocrinol Metab 2015;59(03):277-280. Doi: 10.1590/2359-3997000000051
33. Negri AL, Rosa Diez G, Del Valle E, et al. Hypercalcemia secondary to granulomatous disease caused by the injection of methacrylate: a case series. Clin Cases Miner Bone Metab 2014;11(01):44-48
34. Costa FW, Teixeira LH, Carvalho FS, et al. Bilateral oral nodules after the use of a dermal filler containing polymethylmethacrylate microspheres in an older woman. J Am Geriatr Soc 2014;62 (03):587-588. Doi: 10.1111/jgs.12717
35. Park TH, Seo SW, Kim JK, Chang CH. Clinical experience with polymethylmethacrylate microsphere filler complications. Aesthetic Plast Surg 2012;36(02):421-426. Doi: 10.1007/s00266-011-9803-z
36. Park TH, Yeo KK, Seo SW, et al. Clinical experience with complications of hand rejuvenation. J Plast Reconstr Aesthet Surg 2012;65(12):1627-1631. Doi: 10.1016/j.bjps.2012.06.008
37. Solomon P, Sklar M, Zener R. Facial soft tissue augmentation with Artecoll(®): A review of eight years of clinical experience in 153 patients. Can J Plast Surg 2012;20(01):28-32. Doi: 10.1177/229255031202000110
38. Al-Qattan MM. Complications related to Artecoll injections for soft tissue augmentation of the hand: 3 case reports. J Hand Surg Am 2011;36(06):994-997. Doi: 10.1016/j.jhsa.2011.03.016
39. Santana KP, Pereira LH, Sabatovich O, Sterodimas A. Foreign-body granulomas caused by polymethylmethacrylate (PMMA) microspheres: successful correction by autologous fat transplantation. J Plast Reconstr Aesthet Surg 2010;63(02):e139-e141. Doi: 10.1016/j.bjps.2009.01.088
40. de Figueiredo JCA, Naufal RR, Zampar AG, Mélega JM. Expanded median forehead flap and abbé flap for nasal and upper lip reconstruction after complications of polymethylmethacrylate. Aesthetic Plast Surg 2010;34(03):385-387. Doi: 10.1007/s00266-008-9294-8
41. Wollina U. Facial recontouring for late dermal filler complications. Kosmetische Medizin. 2009;6:219-221
42. Wolfram D, Tzankov A, Piza-Katzer H. Surgery for foreign body reactions due to injectable fillers. Dermatology 2006;213(04): 300-304. Doi: 10.1159/000096193
43. Sidwell RU, McL Johnson N, Francis N, Bunker CB. Cutaneous sarcoidal granulomas developing after Artecoll facial cosmetic filler in a patient with newly diagnosed systemic sarcoidosis. Clin Exp Dermatol 2006;31(02):208-211. Doi: 10.1111/j.1365-2230.2005.01993.x
44. Kates LC, Fitzgerald R. Poly-L-lactic acid injection for HIV-associated facial lipoatrophy: treatment principles, case studies, and literature review. Aesthet Surg J 2008;28(04):397-403. Doi: 10.1016/j.asj.2008.06.005
45. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de DST, Aids e Hepatites Virais. Manual de tratamento da lipoatrofia facial : recomendações para o preenchimento facial com polimetilmetacrilato em portadores de HIV/aids / Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de DST, Aids e Hepatites Virais. Brasília: Ministério da Saúde; 2009
46. Blanco Souza TA, Colomé LM, Bender EA, Lemperle G. Brazilian Consensus Recommendation on the Use of Polymethylmethacrylate Filler in Facial and Corporal Aesthetics. Aesthetic Plast Surg 2018;42(05):1244-1251. Doi: 10.1007/s00266-018-1167-1
47. Cappella A, Galvão L, Nazar L, Alves D (2023). Polimetilmetacrilato na Harmonização Orofacial-Vantagens e desvantagens, Revisão de literatura. Revista Científica da UNIFENAS-ISSN: 2596-3481, 5(1).
48. Li K, Meng F, Li YR, et al. Application of Nonsurgical Modalities in Improving Facial Aging. Int J Dent 2022;2022:8332631. Doi: 10.1155/2022/8332631
49. Anvisa. Anvisa esclarece sobre indicações do PMMA. Ministério da Saúde, publicado em 04/07/2022, atualizado 03/11/2022. Disponível em: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2018/anvisa-esclarece-sobre-indicacoes-dopmma
50. Papazian MF, Silva LM, Crepaldi AA, Crepaldi MLS, Aguiar AP. Principais aspectos dos preenchedores faciais. Rev FAIPE 2018;8 (01):101-116
1. Private Practice, São Paulo, SP, Brazil
2. Medicine Program, Escola Bahiana de Medicina e Saúde Pública, Salvador, BA, Brazil
3. Medicine Program, Universidade Salvador, Salvador, BA, Brazil
4. School of Medicine, Universidade de São Paulo, São Paulo, SP, Brazil
Address for correspondence Alessandra Lima Costa, Curso de Medicina, Escola Bahiana de Medicina e Saúde Pública, Salvador, BA, Brazil (e-mail: alessandracosta21.1@bahiana.edu.br).
Article received: August 14, 2024.
Article accepted: September 29, 2024.
Conflict of Interests
The authors have no conflict of interests to declare.








 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter