

Original Article - Year 2024 - Volume 39 -
Elevated expression of galectin-3 in excessive scars: A pilot study
Elevada expressão de galectina-3 em cicatrizes excessivas: Um estudo piloto
ABSTRACT
Introduction: Hypertrophic scars and keloids are types of excessive scars from abnormal skin healing. Galectin-3 (gal-3) is a protein from the lectin family capable of identifying carbohydrates, which can combine and act on different molecules. In the literature, the action of gal-3 as the main regulatory agent of fibrogenesis has already been described and is currently used in anti-fibrotic therapy for various organs such as the lung and liver. The objective of this pilot study was to show preliminary results found in the expression of gal-3 in exacerbated scars.
Method: Twenty biopsy samples from excessive scars (16 keloids and 4 hypertrophic scars) and 9 samples from normal scars were collected from 22 women and 7 men. These samples were processed for routine histopathological analysis by immunohistochemistry to detect gal-3. Gal-3 positive cells were quantified by the stereological method using a 36-point grid.
Results: Immunohistochemistry showed high expression of gal-3 in endothelial and epithelial cells of all scar samples, as well as expression in cells distributed throughout the dermis. Higher gal-3 expression was found in keloid samples (28% positive cells) compared to normal (18%) and hypertrophic (22%) scars (p=0.0075). The results were obtained from a small number of patients, as this was a pilot study.
Conclusion: The data suggest that gal-3 participates in the healing process and, due to its greater presence in keloid samples, it may be a potential biomarker for keloid formation and a promising therapeutic target to be explored.
Keywords: Immunohistochemistry; Keloid; Cicatrix, hypertrophic; Galectin 3; Wound healing
RESUMO
Introdução: Cicatrizes hipertróficas e queloides são tipos de cicatrizes excessivas de cicatrização anormal da pele. Galectina-3 (gal-3) é uma proteína da família das lectinas capaz de identificar carboidratos, que podem se combinar e atuar em diversas moléculas. Na literatura, a ação da gal-3 como principal agente regulador da fibrogênese já foi descrita, sendo atualmente utilizada na terapia antifibrótica de diversos órgãos como pulmão e fígado. O objetivo deste estudo piloto foi mostrar resultados preliminares encontrados na expressão de gal-3 em cicatrizes exacerbadas.
Método: Foram coletadas 20 amostras de biópsias de cicatrizes excessivas (16 queloides e 4 cicatrizes hipertróficas) e 9 amostras de cicatrizes normais de 22 mulheres e 7 homens. Essas amostras foram processadas para análise histopatológica de rotina por imuno-histoquímica para detectar gal-3. As células positivas para gal-3 foram quantificadas pelo método estereológico utilizando uma grade de 36 pontos.
Resultados: A imuno-histoquímica mostrou alta expressão de gal-3 em células endoteliais e epiteliais de todas as amostras de cicatrizes, bem como expressão em células distribuídas pela derme. Maior expressão de gal-3 foi encontrada em amostras de queloides (28% de células positivas) em comparação com cicatrizes normais (18%) e hipertróficas (22%) (p=0,0075). Os resultados foram obtidos de um pequeno número de pacientes, por se tratar de um estudo piloto.
Conclusão: Os dados sugerem que a gal-3 participa do processo de cicatrização e, devido à sua maior presença em amostras de queloides, pode ser um potencial biomarcador para formação de queloides e um alvo terapêutico promissor a ser explorado.
Palavras-chave: Imuno-histoquímica; Queloide; Cicatriz hipertrófica; Galectina 3; Cicatrização
INTRODUCTION
Tissue repair is defined as the restoration of tissue after injury, both in terms of its conformation and its function1. Healing is an evolutionary process preserved to this day and includes processes such as inflammation, cell proliferation, and remodeling of the extracellular matrix2,3. Incorrect or abnormal tissue repair of the skin causes pathological situations such as keloids and hypertrophic scars. Keloids and hypertrophic scars are forms of excessive scarring, which can be caused by skin injury, burns, trauma, irritation, surgery, and piercing, among other types of skin injuries4.
Exacerbated deposition of extracellular matrix components can occur during the healing phases and, when the remodeling of the lesion shows progression of the matrix, fibrosis occurs. Fibrogenesis can be characterized as dynamic, with a varying degree of plasticity, which also depends on the tissue affected5. The remodeling of fibrotic tissue directly depends on chronic inflammation and damage to tissue cells6. In these pathological situations there is also continuous inflammation, the presence of myofibroblasts, and excessive deposition of collagen, fibroblasts, inflammatory cells, and newly formed blood vessels7,8. To date, the exact cause of these pathological situations is not known, but several treatments are used to induce regression of these excessive scars9. Despite advances in its treatment, many patients still experience adverse effects from abnormal scars9.
Galectins are proteins that belong to the lectin family and to date, 15 different types have been identified. These proteins bind to β-galactoside and have a carbohydrate-binding recognition domain (CRD) with the ability to recognize carbohydrates of approximately 130 amino acids.10.11. A unique form of galectin, galectin-3 (gal-3), called chimera, arises due to a disordered and intrinsic sequence present in its N-terminal domain, which allows the oligomerization and organization of gal-3 into pentamers. Gal-3 has multifunctional capacity in numerous cellular and pathological conditions11.
Several studies have already demonstrated the ability of gal-3 to promote re-epithelialization, angiogenesis, adhesion, and cell migration, both in murine experimental models and in vitro models, including participation in the regulation of the immune system12,13,14,15,16,17,18. The ability to act in cell-matrix interaction makes gal-3 essential for promoting these processes19. Therefore, gal-3 plays roles in several processes, such as scar formation, mediating monocyte recruitment, macrophage differentiation plasticity, intercellular interaction, and matrix production, and, thus, indirectly, in the regulation of fibrosis20,21,22,23.
Several factors may be involved in the development of excessive scarring. Recently, some articles have suggested the involvement of gal-3 in various pathological situations, including fibrosis, such as cardiac fibrosis and pulmonary fibrosis24,25. Furthermore, recent work suggests that gal-3, together with galectin-1, assists in the proliferation and survival of fibroblasts, which are directly responsible for the excessive deposition of scar matrix26. Therefore, more studies are needed to correlate the expression and action of gal-3 in normal and abnormal tissue repair.
OBJECTIVE
The present study aims to investigate and characterize the expression of gal-3 in human samples of hypertrophic scars and keloids, comparing them with normal scars.
METHOD
Patients and Samples
The study was approved by the Research Ethics Committee of the Hospital Universitário Pedro Ernesto, according to protocol number 1,900,610. All patients were informed about the study; participants over 18 years of age who voluntarily consented to participate signed the Free and Informed Consent Form (TCLE), while individuals between 13 and 17 years old signed the Assent Form together with a guardian. Patients who chose not to undergo the surgical procedure to remove scars, children under 13 years of age, or individuals who refused to sign the Informed Consent Form were not included in the study.
Normal scars, keloids, and hypertrophic scars were collected as samples from patients treated in the Plastic Surgery Sector of the Hospital Universitário Pedro Ernesto, in Rio de Janeiro, RJ, from 2015 to 2019, who wished to undergo the procedure to remove these scars, which showed no signs of regression and did not respond to treatment. The classification of normal scars, hypertrophic scars, and keloids was carried out using strict clinical criteria (assessment carried out by a trained doctor).
For surgery, the skin was previously prepared with local antiseptics such as 2% Chlorhexidine gluconate, degreaser, and alcohol. Patients underwent surgical intervention under local anesthesia with 1% lidocaine and 1:200,000 adrenaline. Excision of hypertrophic and unsightly scars was performed in a spindle covering the entire lesion, while in cases of keloid scars, intralesional resection was performed due to the risk of recurrence.
The fragments were fixed in 10% formaldehyde for at least 24 hours. After fixation in formalin, the fragments were processed in paraffin and transferred to histological slides for histopathological and immunohistochemical analysis. For general tissue analysis, the samples were stained with hematoxylin and eosin (HE) and, to observe the collagen fiber system, the samples were stained using the Picrosirius Red (PS) technique. The HE and PS sections were visualized under a Leica DM 500 microscope, under a bright field, or with a polarizer, respectively. After capturing images of histological sections, analyses were performed using the Future WinJoe capture program version 1.6 (Future Optics Sci. & Tech. Co, Xiasha, Hangzhou, China).
Immunohistochemistry
To identify myofibroblasts, cells important in tissue repair and fibrosis, as well as tissue vascularization, the immunohistochemistry technique was performed using an antibody against alpha-smooth muscle actin (α-SMA; Abcam-UK).
In this methodology, endogenous peroxidase was blocked in a 3% hydrogen peroxide solution (Proquímios-BR) in methanol for 30 minutes. This was followed by 5-minute washes in running water and 1x phosphate-buffered saline (PBS), after which samples were blocked using the SpringBio blocking kit. After 1 hour, the excess blocking solution was removed and sections were incubated with mouse anti-α-SMA primary monoclonal antibody at a concentration of 1/100 in 1% bovine serum albumin (BSA) in PBS at 4°C at night; at this stage, the negative control slides were incubated only with PBS/BSA 1%.
In the second step, the primary antibody was washed with 1x PBS, then incubated with anti-mouse IgG secondary antibody - HighDef Complement Kit (Cell Marque-USA) for 30 minutes. The labeling was developed with the chromogen diaminobenzidine (DAB; Cell Marque-USA) for 5-8 minutes. The sections were washed again with 1x PBS and counterstained with Harris hematoxylin (Proquímios-BR). The slides were washed in running water, then in distilled water, and then dehydrated, clarified, and mounted with Entellan (Merck-Ger). The slices were viewed under a bright-field microscope (Leica DM500).
To identify the presence of gal-3 in scar samples, immunohistochemistry was performed using the anti-gal-3 primary monoclonal antibody (Clone M3/38; American Type Culture Collection-USA). Samples were deparaffinized and rehydrated and antigen retrieval was performed with Trilogy® solution (1:100 diluted in distilled water; Sigma-USA). The slides were then passed in a distilled water bath followed by a 3% hydrogen peroxide solution to block endogenous peroxidase. The slides were washed again in a distilled water bath and 3 baths of PBS+Tween (10%), followed by blocking the specific site with 16% milk and 20% BSA in distilled water for 1 hour.
Sections were then incubated with anti-gal-3 antibody at a concentration of 1:100 in PBS/BSA 1% for 1 hour; At this stage, the slides for the negative control were incubated only with PBS/BSA 1%. Slides were washed three times with PBS + Tween followed by incubation with an unconjugated anti-mouse IgG secondary antibody (Vector-USA). Three washes were performed with PBS + Tween and then, to amplify the secondary antibody signal, streptavidin peroxidase (Sigma-USA) was added for 20 minutes. Sections were washed again with PBS+Tween and DAB chromogen was added for 20 seconds. Contrast was performed with Harris hematoxylin (Sigma-USA). Finally, dehydration and clarification were performed, and the slides were mounted with Entellan and viewed under a microscope (Leica DM 500).
Galectin-3 quantitative analysis and image capture
Three random fields from each slide were imaged for the epidermis, dermis, and subcutaneous tissue. After capturing images of the histological sections, analyses were performed using the Future WinJoe capture program.
Future WinJoe software was used to process the captured digital images and the volume density of gal-3 positive cells (%Vv [gal-3]) was evaluated using a stereological system consisting of 36 points, as previously described by Petito et al.27. Vv = PP/PT (%) (PP represents the evaluated points that reach the structure and PT represents the total number of points present in the grid). The results were expressed as mean ± standard deviation.
RESULTS
Scars
The patients were subdivided into three groups (normal scars, hypertrophic scars, and keloids) to allow statistical analysis, as shown in Table 1. All subjects in the normal scar group were women (n=9) and aged between 20 and 57 (average 37.3 years). The majority of normal scars were located in the breast (18%) and pelvic region (18%), followed by the lower abdomen (9%), navel (9%), and hypochondrium (9%). In the keloid group (n=16) the majority of patients were women (62.5%) and aged between 13 and 66 (average 28.3 years). And most of the keloids were collected in the ears (50.0%), followed by the abdomen (12.4%), anterior trunk (12.4%), cervical region (6.2%), scalp (6. 2%), superciliary region (6.2%) and mandible (6.2%). Finally, the group with hypertrophic scars (n=4) was composed of 3 women and 1 man, aged between 21 and 61 (average 34.25 years). And these scars were collected from each umbilical, pre-auricular, face, and shoulder area. The average duration of typical scars was 65 months, while keloids averaged 83 months and hypertrophic scars averaged 30 months.
| Normal scar (N = 09) | Keloid (N = 16) | Hypertrophic Scar (N = 04) | |
|---|---|---|---|
| Years | 37.33±12.76 | 28.37±15.31 | 34.25±18.46 |
| Sex | 09 (100%) F | 10 (62.5%) F | 03 (75%) F |
| 00 (0%) M | 06 (37.5%) M | 01 (25%) M | |
| Skin color * | |||
| White | 07 | 01 | 01 |
| Brown | 02 | 11 | 01 |
| Black | 00 | 02 | 01 |
| S.D. | 00 | 02 | 01 |
Stained samples
HE-stained samples from all scars revealed a small number of cells in the reticular dermis, demonstrating greater deposition of the extracellular matrix. Furthermore, keloids and hypertrophic scars had straighter epidermis and fewer dermal papillae compared to normal scars (Figure 1A, B, and C).
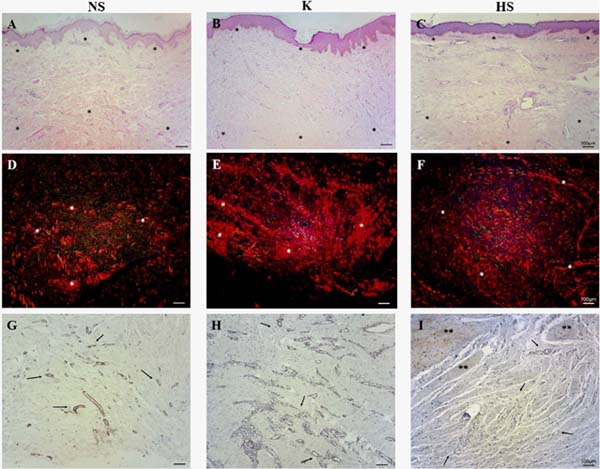
Under the polarization microscope, it was possible to observe that all samples had thick, reddish fibers, demonstrating a large deposition of collagen in the reticular dermis. The collagen fibers in a normal scar tend to be fragmented and intertwined. Keloids and hypertrophic scars have longer and thicker fibers, deposited parallel to the surface, providing more exacerbated, irregular, and stratified fibrous deposition (Figure 1D, E, and F).
α-SMA expression in excessive scar samples
Tissue samples from normal scars, keloids, and hypertrophic scars showed considerable positive staining for α-SMA in blood vessel walls; however, normal scars showed less vascularity throughout the cut, compared to keloids and hypertrophic scars, especially in the reticular dermis region (Figure 1G, H and I).
Galectin-3 expression in excessive scar samples
In general, gal-3 expression was observed in the epidermis and throughout the papillary dermis and areas of the reticular dermis. Its staining was more intense in cells of blood vessels and attached glands, but gal-3 was not present in regions with deposition of collagen fibers. In normal scars, positive staining for gal-3 was observed throughout the tissue, mainly in the papillary dermis, while in the reticular dermis, it was not possible to detect relevant staining in the regions of fiber deposition, only positivity around the blood vessels and sweat glands (Figure 2A, B and C).
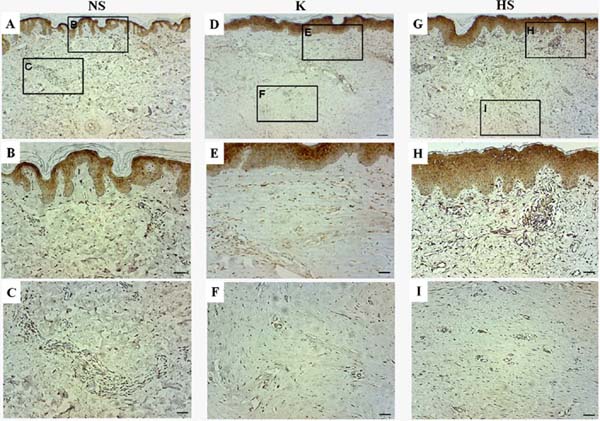
In keloids, positive staining was observed throughout the epithelium and in the papillary dermis, mainly in the blood vessels of the papillary and reticular dermis. Furthermore, the keloid group showed the highest staining of gal-3-positive cells, however, no positive staining was observed in sites with excessive extracellular matrix deposition (Figures 2D, E, and F). In hypertrophic scars, positive staining for gal-3 was also observed in the blood vessels of the papillary and reticular dermis, but no positive staining was observed in the region of extracellular matrix deposition. In general, the hypertrophic scar group showed a similar pattern to the keloid group, but with a lower number of gal-3-positive cells (Figure 2G, H, and I).
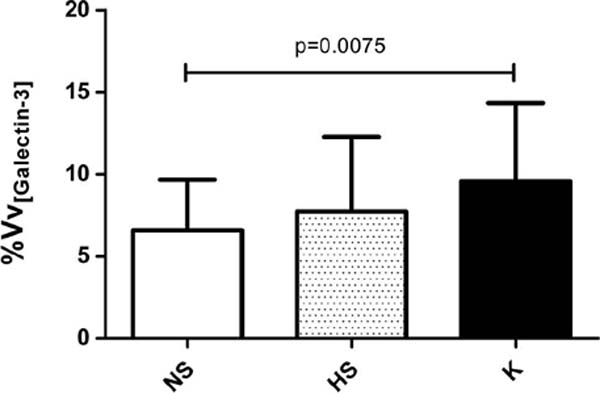
As the distribution pattern of gal-3 was similar between the groups studied, cells positive for gal-3 were then quantified by stereology. By determining and comparing the expression of gal-3 in cells distributed throughout the dermis, it was possible to observe a significant difference in the volume density of gal-3-positive cells in keloids compared to normal scars (p = 0.0075) (Figure 3). However, there was no statistically significant difference between the volume density of gal-3-positive cells between hypertrophic and normal scars, nor between keloids and hypertrophic scars. Samples from the keloid group showed 28% of cells positive for gal-3, while the group with normal scars showed 18% and the group with hypertrophic scars 22% (Figure 3).
DISCUSSION
Galectin-3 has been described as having a role in inflammatory and angiogenic processes16,28 and is therefore implicated in wound healing processes, but its role has not yet been specifically defined. It is also not yet known how keloids and hypertrophic scars arise; The present study aimed to characterize the expression of gal-3 in normal scars and excessive scars in humans.
Healing is a type of repair mechanism that is activated when tissue damage occurs. On some occasions, the healing process of skin wounds does not occur normally, leading to the formation of excessive scars, such as keloids and hypertrophic scars. This excessive scarring is characterized by persistent inflammation, with unbalanced cell recruitment and remodeling, which leads to excessive deposition of matrix components, resulting in fibrosis5. Tissue fibrosis is dependent on key mediators and specific molecular pathways, therefore, fibrosis can occur in completely different organs, just by the presence of these mediators and molecular pathways6.
Galectins are proteins known to have a carbohydrate-binding recognition domain (CRD). Type 3 galectin is the most different of the 17 known galectins and is best known for having interaction domains with proteins and carbohydrates, in addition to the ability to pentamerize, allowing different interactions with molecules and tissues11. According to Peiró et al.6, gal-3 is related to the fibrogenesis pathway, including cutaneous fibrosis, corroborating the data found in our study, in which we quantified more gal-3 in fibrotic tissue, such as keloids. Furthermore, according to the literature, gal-3 is also related to angiogenesis and macrophage recruitment16,21,23. Therefore, it is understood that this protein is directly linked to the healing process. Also, other galectins have already been studied regarding their role in tissue repair, such as galectin-1, which has been suggested as having therapeutic potential, as its subcutaneous application in wound sites accelerated healing29.
Arciniegas et al.26 demonstrated the expression of gal-3 in the basal lamina and the dermal/epidermal interface, as well as in some immune cells, microvessels, collagen bundles, and fibroblasts in keloid tissues, which agrees with our data but does not compare the expression of gal-3 with tissue samples from normal scars and hypertrophic scars.
The data presented by Amadeu et al.7 and agreed by Tan et al.8 highlighted the difference between the vascularization of normal scars and abnormal scars, hypertrophic scars, and keloids, in which the tissue of excessive scars was more vascularized. This difference in vascularization corroborated the data found in our study for the expression of the smooth muscle markers α-SMA and gal-3 in normal and excessive scars. Our work also found that blood vessels were more present in excessive scars and that there was greater expression of gal-3 in the blood vessels of excessive scars, in addition to greater expression of gal-3 in keloids in general.
The findings of Mostacada et al.28 suggested that gal-3 is vital for the recruitment of macrophages, influencing their eliminative action against apoptotic cells and microorganisms. A decrease in gal-3 therefore leads to a less pronounced inflammatory response, due to less recruitment of pro-inflammatory cells. Furthermore, Sciacchitano et al.11 demonstrated the ability of gal-3 to mediate the activation of IL- 4-induced macrophages, known as alternatively activated macrophages, to stimulate fibrosis and matrix production. These data could explain the higher expression of gal-3 in keloids compared to normal scars, but further studies are needed to fully understand the higher expression of gal-3 in keloids compared to hypertrophic scars.
It has not yet been possible to induce excessive scarring in animal models, therefore studies must be based on human samples. The present study was limited due to the scarce number of samples, mainly from hypertrophic scars, which probably impacted the statistical analysis when comparing gal-3 expression between groups. Furthermore, due to this small number of samples, only tissue analysis was performed and no investigation of gal-3 in the fibrogenesis pathway could be performed. Furthermore, the cuts already had a complete healing structure, therefore, the period of cellular modulation during healing could not be evaluated. The absence of normal skin samples was our choice because this study aims to compare two types of excessive scarring in human skin (hypertrophic scars and keloids) with the normal wound healing process (normal scars). Despite the limitations of this study, the results obtained are valuable for a pilot study.
CONCLUSION
In this study, gal-3 protein expression was detected in normal and abnormal scars, suggesting its involvement in typical and atypical cutaneous wound healing processes, particularly angiogenesis and re-epithelialization. Despite the heterogeneity of the normal and abnormal scar samples used in this study, gal-3 was identified and its expression showed significant differences. Furthermore, its expression was notably higher in the dermis of keloids compared to typical scars, indicating a fundamental role of gal-3 in keloid formation. Consequently, further investigations are needed to evaluate the deposition and function of gal-3 throughout the healing cascade and in chronic conditions such as diabetes and ischemic injuries. Such studies may reveal the potential of gal-3 as a biomarker for keloid formation or as a target for therapeutic interventions in wound treatment.
ACKNOWLEDGMENT
The authors would like to thank Elaine N. Silva, Igor Rodrigues, Bárbara Dantas, Flavia Loureiro, and Gabriel Marujo for the technical support and collection of patient samples, respectively.
REFERENCES
1. van Dongen JA, Harmsen MC, van der Lei B, Stevens HP Augmentation of Dermal Wound Healing by Adipose Tissue-Derived Stromal Cells (ASC). Bioengineering (Basel). 2018;5(4):91.
2. Seifert AW, Monaghan JR, Voss SR, Maden M. Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS One. 2012;7(4):e32875.
3. Richardson R, Slanchev K, Kraus C, Knyphausen P, Eming S, Hammerschmidt M.Adult zebrafish as a model system for cutaneous wound-healing research. J Invest Dermatol. 2013;133(6):1655-65.
4. Ogawa R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int J Mol Sci. 2017;18(3):606.
5. Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138-49.
6. Peiró T, Alonso-Carpio M, Ribera P, Almudéver P, Roger I, Montero P, et al. Increased Expression of Galectin-3 in Skin Fibrosis: Evidence from In Vitro and In Vivo Studies. Int J Mol Sci. 2022;23(23):15319.
7. Amadeu T, Braune A, Mandarim-de-Lacerda C, Porto LC, Desmoulière A, Costa A. Vascularization pattern in hypertrophic scars and keloids: a stereological analysis. Pathol Res Pract. 2003;199(7):469-73.
8. Tan Y, Zhang M, Kong Y, Zhang F, Wang Y, Huang Y, et al. Fibroblasts and endothelial cells interplay drives hypertrophic scar formation: Insights from in vitro and in vivo models. Bioeng Transi Med. 2023;9(2):e10630.
9. Oliveira GV, Metsavaht LD, Kadunc BV, Jedwab SKK, Bressan MS, Stolf HO, et al. Treatment of keloids and hypertrophic scars. Position statement of the Brazilian expert group GREMCIQ. J Eur Acad Dermatol Venereol. 2021;35(11):2128-42.
10. Bouffette S, Botez I, De Ceuninck F. Targeting galectin-3 in inflammatory and fibrotic diseases. Trends Pharmacol Sci. 2023;44(8):519-31.
11. Sciacchitano S, Lavra L, Morgante A, Ulivieri A, Magi F, De Francesco GP, et al. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int J Mol Sci. 2018;19(2):379.
12. Ochieng J, Green B, Evans S, James O, Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta. 1998;1379(1):97-106.
13. Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, et al. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277(44):42299-305.
14. Paret C, Bourouba M, Beer A, Miyazaki K, Schnölzer M, Fiedler S, et al. Ly6 family member C4.4A binds laminins 1 and 5, associates with galectin-3 and supports cell migration. Int J Cancer. 2005;115(5):724-33.
15. Saravanan C, Cao Z, Head SR, Panjwani N. Detection of differentially expressed wound-healing-related glycogenes in galectin-3-deficient mice. Invest Ophthalmol Vis Sci. 2009;50(12):5690-6.
16. Funasaka T, Raz A, Nangia-Makker P. Galectin-3 in angiogenesis and metastasis. Glycobiology. 2014;24(10):886-91.
17. Walker JT, Elliott CG, Forbes TL, Hamilton DW. Genetic Deletion of Galectin-3 Does Not Impair Full-Thickness Excisional Skin Healing. J Invest Dermatol. 2016;136(5):1042-1050.
18. Oliveira FL, Bernardes ES, Brand C, dos Santos SN, Cabanel MP, Arcanjo KD, et al. Lack of galectin-3 up-regulates IgA expression by peritoneal B1 lymphocytes during B cell differentiation. Cell Tissue Res. 2016;363(2):411-26.
19. Panjwani N. Role of galectins in re-epithelialization of wounds. Ann Transi Med. 2014;2(9):89.
20. Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, et al. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J Immunol. 2004;173(5):3425-31.
21. Lemos FS, Pereira JX, Carvalho VF, Bernardes ES, Chammas R, Pereira TM, et al. Galectin-3 orchestrates the histology of mesentery and protects liver during lupus-like syndrome induced by pristane. Sci Rep. 2019;9(1):14620.
22. Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4(8):583-94.
23. Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12(1):99-106.
24. Dong R, Zhang M, Hu Q, Zheng S, Soh A, Zheng Y, et al. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int J Mol Med. 2018;41(2):599-614.
25. Nangia-Makker P, Hogan V, Balan V, Raz A. Chimeric galectin-3 and collagens: Biomarkers and potential therapeutic targets in fibroproliferative diseases. J Biol Chem. 2022;298(12):102622.
26. Arciniegas E, Carrillo LM, Rojas H, Ramírez R, Chopite M. Galectin-1 and Galectin-3 and Their Potential Binding Partners in the Dermal Thickening of Keloid Tissues. Am J Dermatopathol. 2019;41(3):193-204.
27. Petito RB, Amadeu TP, Pascarelli BM, Jardim MR, Vital RT, Antunes SL, et al. Transforming growth factor-β1 may be a key mediator of the fibrogenic properties of neural cells in leprosy. J Neuropathol Exp Neurol. 2013;72(4):351-66.
28. Mostacada K, Oliveira FL, Villa-Verde DM, Martinez AM. Lack of galectin-3 improves the functional outcome and tissue sparing by modulating inflammatory response after a compressive spinal cord injury. Exp Neurol. 2015;271:390-400.
29. Lin YT, Chen JS, Wu MH, Hsieh IS, Liang CH, Hsu CL, et al. Galectin-1 accelerates wound healing by regulating the neuropilin-1/Smad3/NOX4 pathway and ROS production in myofibroblasts. J Invest Dermatol. 2015;135(1):258-68.
1. Universidade do Estado do Rio de Janeiro,
Patologia Geral, Laboratório de Imunopatologia, Rio de Janeiro, RJ,
Brazil.
2. Universidade Federal do Rio de Janeiro,
Instituto de Ciências Biomédicas, Rio de Janeiro, RJ, Brazil.
3. Universidade do Estado do Rio de Janeiro, Setor
de Cirurgia Plástica, Rio de Janeiro, RJ, Brazil.
4. Fiocruz, Laboratório de Imunofarmacologia, Rio
de Janeiro, RJ, Brazil.
Corresponding author: Thaís Porto Amadeu Av. Professor Manoel 444, 4oº andar, Maracanã, Rio de Janeiro, RJ, Brazil CEP: 20550-170 E-mail: tpamadeu@gmail.com
Article received: March 05, 2024.
Article accepted: April 30, 2024.
Conflicts of interest: none.





 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter