

Review Article - Year 2024 - Volume 39 -
Use of botulinum toxin for the treatment of keloid scars: scoping review
Utilização da toxina botulínica para o tratamento de cicatriz queloide: revisão de escopo
ABSTRACT
Introduction: Visible scars can cause problems, whether aesthetic, psychological, functional, or social, mainly of great extension and volume, such as keloids. The discovery of new treatments for keloids is not easy, given the presence of some methodological and ethical obstacles, and it is an area that is little explored. Botulinum toxin has been presented as a therapeutic alternative in national and international studies, requiring a compilation and highlighting of the main studies that can support clinical practice. Thus, the objective was to present a scoping review on the therapeutic use of botulinum toxin for the treatment of keloid scars.
Method: The review was carried out using the PICO strategy and using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews. It was carried out in the PubMed/ Medline, Virtual Health Library, and SciELO databases, considering studies from 2016 to September 2021.
Results: Overall, 34 articles related to the topic were found. After filtering and selection, the review was constructed with the support of 5 articles. The studies varied between cohorts, case reports, randomized clinical trials, and casecontrol. It was possible to observe as main results of the short-term action of botulinum toxin in reducing keloids, greater effectiveness in reducing symptoms, and possibilities of clinical use for different populations and clinical manifestations.
Conclusion: The mechanism of action of botulinum toxin can facilitate the treatment of keloids and reduce symptoms, requiring more robust studies to define effective scar management protocols.
Keywords: Keloid; Therapeutic Human experimentation; Botulinum toxins, type A; Reconstructive surgical procedures; Evidence-based practice
RESUMO
Introdução: Cicatrizes visíveis podem acarretar agravos, sejam estéticos, psicológicos, funcionais ou sociais, principalmente de grande extensão e volume, como os queloides. A descoberta de novos tratamentos de queloides não é fácil, visto a presença de alguns entraves metodológicos e éticos, sendo uma área pouco explorada. A toxina botulínica tem sido apresentada como alternativa terapêutica em estudos nacionais e internacionais, sendo necessária uma compilação e destaque dos principais estudos que possam subsidiar a prática clínica. Assim, o objetivo foi apresentar uma revisão de escopo sobre a utilização terapêutica da toxina botulínica para o tratamento de cicatrizes queloides.
Método: A revisão foi realizada através da estratégia PICO e utilizando o Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews. Foi realizada nas bases de dados PubMed/ Medline, Biblioteca Virtual em Saúde e SciELO, considerando estudos do período de 2016 até setembro de 2021.
Resultados: Foram encontrados 34 artigos no geral relacionados ao tema. Após filtragem e seleção, a revisão foi construída com apoio de 5 artigos. Os estudos variaram entre coorte, relatos de caso, ensaio clínico randomizado e caso-controle. Foi possível observar como principais resultados a ação a curto prazo da toxina botulínica na redução de queloides, maior efetividade na redução dos sintomas e possibilidades de utilização clínica para diferentes populações e manifestações clínicas.
Conclusão: O mecanismo de ação da toxina botulínica pode facilitar o tratamento de queloides e redução de sintomas, sendo necessários estudos mais robustos para definição de protocolos cínicos de gestão de cicatrizes.
Palavras-chave: Queloide; Experimentação humana terapêutica; Toxinas botulínicas tipo A; Procedimentos cirúrgicos reconstrutivos; Prática clínica baseada em evidências
INTRODUCTION
Scars are generally a matter of concern for patients undergoing surgical procedures, especially if they are likely to appear in more visible areas of the body1. Surgical wounds in regions such as the face tend to have a greater aesthetic impact during the healing process, and there may also be greater tension in the surgical wound in some myofascial structures, resulting in scars2.
Keloid is characterized as a scar of considerable thickness, raised, resulting from the abnormal growth of scar tissue, which, unlike hypertrophic scars, extends beyond the limits of the surgical wound3. Due to their physiology, keloids do not develop in animals, which makes the process of developing new therapies difficult, as testing on animals cannot be carried out1. Furthermore, the presence of scars can lead to biopsychosocial repercussions, whether physiological or social limitations due to aesthetics4. With these aspects in mind, several treatments are used to reduce these problems.
There is still no consensus on a single treatment that is considered the best alternative for keloid scars. The Virtual Health Library5, with support from the Sociedade Brasileira de Cirurgia Dermatológica and the Sociedade Brasileira de Dermatologia, presents the main treatments for keloids: local radiotherapy, silicone plates, drug injections, occlusive tapes, surgery, cryotherapy, and laser therapy. These treatment options mainly aim to reduce symptoms, with their regression or reduction being less frequent alternatives that are still being studied.
Aiming for better therapy, research using botulinum toxin is gaining more and more space. Botulinum toxin type A (BTA) acts to reduce tension at the edges of surgical wounds during the healing process, thus contributing to improving the scar aspect, and reducing the possibilities of development and/or progression of keloids6.
OBJECTIVE
With this in mind, the present study aims to present a scoping review on the therapeutic use of botulinum toxin for the treatment of keloid scars.
METHOD
The PICO model, based on Santos et al.7, was used to formulate the guiding questions of this study, considering: (P) studies that considered patients with keloid scars, (I) studies in which the main objective was to perform or describe interventions and strategies using botulinum toxin for these patients, (C) studies with or without a control group, (O) studies that reported the development and results of interventions in the short, medium and long term. Studies carried out until September 2021 were included in this review if they met the PICO criteria.
The review was constructed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews: PRISMA-ScR8. The search was carried out in the PubMed/Medline, Virtual Health Library (VHL), and SciELO databases to identify articles on the treatment of keloid scars with botulinum toxin. The search was carried out by combining the terms “botulinum toxin”, “keloid”, “scar” and “treatment”. The terms were used in combination, according to the order mentioned above. The terms are based on descriptors present in the Health Sciences Descriptors (DECs).
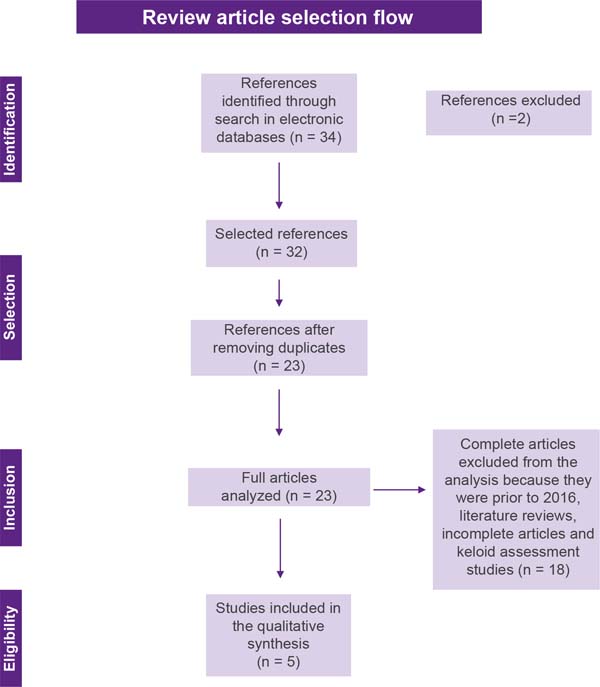
Articles of any design, except reviews, in any language were considered, as long as they were related to the central theme. The exclusion criteria were: unpublished reports, literature reviews, symptom assessment studies, articles published in the period before 2016, and studies with no full text. Articles that met the eligibility criteria were selected based on title and abstract by two reviewers and articles that did not meet the inclusion criteria were excluded. After title and abstract screening, studies were submitted to a public reference manager (Mendeley v.1.17.9) to eliminate duplicates. The result of this selection can be seen in Figure 1.
Subsequently, the remaining full-text articles were examined by a third reviewer. Any disagreement was resolved through discussion until consensus was reached, or with the involvement of a fourth reviewer. Then, the following points were extracted from each study, when available: authorship, year of publication, title, objectives, and results. These data were arranged in tables in Microsoft Word 2016, for final inclusion analysis.
RESULTS
The initial literature search found 34 studies. Of these, 12 studies were identified using PubMed/Medline, 20 using the VHL, and 2 in SciELO. After selection by title and abstract, 32 articles were run in Mendeley to eliminate duplicates. The resulting 23 full-text articles were reviewed to establish whether the publication met the inclusion criteria and 5 were considered eligible (Figure 1).
Of the 5 articles eligible for this review, 1 is a cohort study, 2 are case reports, 1 is a randomized clinical trial and 1 is a case-control study (Chart 1). The search strategy and study inclusion and exclusion criteria are detailed in Figure 1.
| Reference | Title | Study type |
|---|---|---|
| Cardoso et al. (2016)2 | Application of botulinum toxin in secondary intention healing | Case report |
| Pruksapong et al. (2017)9 | Efficacy of Botulinum Toxin A in Preventing Recurrence of Keloids: Double Blinded Randomized Controlled Trial Study: Intraindividual Subject | Randomized Clinical Trial |
| Zhou et al. (2017)10 | Evaluation on efficacy and adverse reactions of combined therapy with botulinum toxin type A in treatment of keloid | Case-control |
| Rasaii et al. (2019)11 | Intralesional triamcinolone alone or in combination with botulinium toxin A is ineffective for the treatment of formed keloid scar: A double blind controlled pilot study | Cohort |
| Pires et al. (2020)12 | Botulinum toxin
type A in the treatment of hypertrophic burn scars in pediatric
age: Clinical Case |
Case report |
Source: Production of the authors.
Regarding treatments, the studies present variations concerning their populations, methods, and clinical criteria, as shown in Chart 2.
| Reference | Method | Clinical criteria |
|---|---|---|
| Cardoso et al. (2016)2 | N=1 Age = 36 years Botulinum toxin application type A |
Scar resulting
from Mohs micrographic surgery in the supralabial region;
Operative scar measuring 3 x 1.6cm; Application in the immediate postoperative period |
| Pruksapong et al. (2017)9 | N=25 patients, 50 keloids Average age = 26 years Control group = injection of corticosteroid therapy Study group = toxin botulinum type A |
Present two scars keloids; Not being pregnant or breastfeeding; Scars larger than 10cm; not be allergic to toxin, lidocaine; Do not present undesirable medical conditions and use anticoagulant drugs or antiplatelet agents |
| Zhou et al. (2017)10 | N=58 Control group = injection of betamethasone and topical hyaluronic acid Study group = toxin combined type A botulinum with injection of betamethasone and topical hyaluronic acid |
Present scars
keloids; No restrictions on the pharmacological components of the study |
| Rasaii et al. (2019)11 | N=23 Average age = 23 years Control group = intralesional triamcinolone acetonide plus placebo Study Group = botulinum toxin type A combined with saline solution |
Present two scars keloids; Do not be pregnant or breastfeeding; Absence of neuromuscular junction disease or use of neuromuscular junction blockers |
| Pires et al. (2020)12 | N=1 Age = 12 years Application of botulinum toxin type A with prior topical analgesia with lidocaine + prilocaine 25 mg/g cream |
Scars resulting
from 2nd and 3rd burns on the face, scalp,
ear pinna, neck, anterior side of the chest, and upper limbs;
Application to the right axillary scar and radial border of the first finger of the right hand; Application 5 months post-burn |
Source: Production of the authors.
The selected studies present different outcomes and conclusions regarding the use of BTA. In Chart 3 it is possible to observe the treatments used, results, and conclusions of the studies analyzed.
| Reference | Treatment | Results | Conclusions |
|---|---|---|---|
| Cardoso et al. (2016)2 | 8 units of BTA in the surgical wound, with healing by secondary intention. | Complete wound healing in 18 days with formation of slightly erythematous scar tissue on the upper lip, with slight extension to the supralabial region, maintained in the late postoperative period, favoring aesthetics and functionality. | The molecular properties of BTA suggest that its action is best at the beginning of healing, when the fibroblasts are still in the proliferative phase and have intense apoptotic activity, requiring further studies on this process in secondary healing. |
| Pruksapong et al. (2017)9 | Control group = Injection of triamcinolone
acetonide (10mg/cc) seven days after stitch removal, repeated in
the first, second, and third months. Study group = intradermal BTA, with a dose of 1.5 units / 1cm length (Botox® 50 units of toxin with 0.9% NSS for injection 2.5cc, concentration 2 units per 0.1cc) seven days after stitch removal (one dose). |
In the first and third months, the outcome in the toxin group was more favorable than in the control group, while the outcome in the control group was more favorable than in the toxin group in the sixth month of follow-up. | The use of BTA is significantly better at preventing keloids from recurring when compared to corticosteroid therapy after one and three months. However, corticosteroid therapy offers significantly better results at 6-month follow-up. |
| Zhou et al. (2017)10 | Control group =
Betamethasone and topical hyaluronic acid
injection. Study group = botulinum toxin type A combined with betamethasone and topical hyaluronic acid injection. Both groups of patients were locally injected with betamethasone once every 4 weeks, 3 consecutive times, and topical hyaluronic acid was used daily. Patients in the combined treatment group were injected with botulinum toxin type A into the periphery of the skin lesion after the first injection of betamethasone. |
The aesthetics
of the skin lesions in the study group improved better after 3
applications. Pain and itch scores in the control group
decreased during 1 month of treatment but gradually increased at
2 and 3 months; in contrast, in patients in the study gradually
decreased within 3 months of treatment, this difference being
statistically significant. Over the course of 3 months of treatment, the thickness of skin lesions in the study group gradually decreased, but the reduction in the control group was not significant. The pain, itching symptoms, and skin thickness of patients in the control group increased 2 weeks after each injection, while the combination treatment group did not experience recurrence. The incidence of adverse reactions in the control group was 26.7% and in the study group it was 25.0%, with no significant difference between the two groups. |
BTA combined with local injection of betamethasone and topical hyaluronic acid for the treatment of keloids is more effective than local injection of betamethasone and topical hyaluronic acid alone, and there is no significant difference in the incidence of adverse reactions, being favorable for clinical application. |
BTA: botulinum toxin type A. Source: Production of the authors.
DISCUSSION
Aesthetic and functional issues related to scars, especially keloids, end up generating discomfort and dissatisfaction on the part of people who have them. Studies such as that by Motoki et al.13 present negative results from interviewees concerning dimorphic disorders of the self-concept and the body, and also state that people with keloids in socially more seen regions such as the face, chest, and upper limbs report a greater negative impact on their body image.
The development of treatment strategies for this condition is a challenge for the scientific community since keloids do not develop in animals, which limits the possibilities for research and testing of new therapeutic elements1. As possibilities, the clinical and scientific community uses strategies already tested in other conditions, in addition to studies with in vitro cells to generate new options14. Lee et al.15 present combined therapy as the main alternative in the treatment of keloids, whether this therapy involves lasers, cryotherapy, or intralesional drug injection, presenting greater safety and efficacy when compared to individual monotherapies.
As an emerging therapeutic, the use of botulinum toxin type A is gaining increasing attention from the clinical and scientific communities. This greater interest can be observed when this work initially found 34 studies that related the use of BTA for the treatment of keloids. In this review, five studies were eligible, ranging from cohort studies, case reports, randomized clinical trials, and case-control studies. Due to the previously mentioned difficulties in developing new research in the area, there is still little variation in the types of studies, which can be seen as an obstacle to treating the condition.
The studies analyzed present variations in their populations and clinical criteria, but it is possible to observe that in two studies10,11 combined therapy was used, as advocated by Lee et al.15, these two studies presented results favorable to the replication of combined therapy for treatment of keloid. Concerning samples and methodologies, the absence of application protocols and considerable methodological deficiencies can be highlighted, mainly regarding sample size and uniformity of treatments.
It was also observed that the studies highlighted the exclusion of participants who are allergic to the components of the treatment, pregnant and lactating women, who use anticoagulant or antiplatelet drugs, as well as those with neuromuscular junction disease or the use of neuromuscular junction blockers, being the effectiveness of treatment with BTA has not been tested in these populations, therefore, without indication of scientific evidence and clinical replicability for them9-11.
The five studies applied BTA intradermally, either at the edge of the scar/operative wound or directly at the site. Sohrabi & Goutos16 add to these studies when they state, in their review, that other research also points to the application of BTA in keloids as a growing treatment to minimize tension on the scar edge and optimize the activity of fibroblasts, directly implicated in the pathogenesis of the formation of scars. In the present study, variation in the dosage of BTA was still observed. As it is composed of studies with different populations, ages, and clinical conditions, this review was unable to define a dosage standard for the toxin, as this dosage is linked to and dependent on the clinical manifestation, size of the scar, and the event that triggered the healing process.
Despite these variations, the five studies observed are related to the findings resulting from BTA-based therapy. It is possible to highlight as the main results the acceleration of the healing process of surgical wounds and reduction of scar formation2, significant short-term results in the reduction of keloids9, and improvement and maintenance of aesthetic, functional, and symptomatic aspects, especially pain and itching10-12. In other reviews5,16 it was also possible to find the replicability of these results, with the use of BTA for keloid a possibility being of effective clinical treatment and with evidence already presented in clinical and scientific circles.
The use of botulinum toxin type A for the treatment of keloid scars is justified mainly by its chemoimmobilizing mechanisms of the muscles in the region, and its action on fibroblastic activity. Studies conclude that the use of botulinum toxin type A has a better effect at the beginning of the healing process, with direct action on fibroblasts2, that this treatment presents fewer adverse reactions and better short-term results when compared to other injectable pharmacological treatments9,10, that this alternative acts better in managing symptoms in different populations and clinical manifestations5, even in pediatric age16, thus encouraging the clinical community to consider BTA as a therapeutic alternative for selected and well-analyzed cases of keloids, always taking into account the clinical particularities and manifestation of the condition.
CONCLUSION
The most recent studies suggest a good potential for the use of botulinum toxin type A for the treatment of keloid scars, mainly for short-term results, and reduction of local symptoms such as pain and itching when compared to other pharmacological treatments. However, there are deficiencies in the studies as they have small populations, short follow-up periods, and lack of homogeneity in the results found. Therefore, it is necessary to develop larger studies with better methodologies, aiming to better define the use of BTA for the treatment of keloids in different situations, and the development of unified scar management protocols for better clinical replicability.
REFERENCES
1. Ogawa R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int J Mol Sci. 2017;18(3):606.
2. Cardoso AS, Teixeira DA, Oliveira BV, Carneiro PP, Junqueira RF. Botulinum toxin application in the secondary intention healing. Surg Cosmet Dermatol 2016;8(2):163-6.
3. Hochman B, Farkas CB, Isoldi FC, Ferrara SF, Furtado F, Ferreira LM. Distribuição de queloide e cicatriz hipertrófica segundo fototipos de pele de Fitzpatrick. Rev Bras Cir Plást. 2012;27(2):185-9.
4. Gouveia BN, Ferreira LD, Rocha Sobrinho HM. O uso da toxina botulínica em procedimentos estéticos. Rev Bras Mil Ciênc. 2020;6(16):56-63.
5. Brasil. Ministério da Saúde. Biblioteca Virtual em Saúde. Queloide. 2021. Elaborado por: Sociedade Brasileira de Cirurgia Dermatológica e Sociedade Brasileira de Dermatologia. Brasília: Ministério da Saúde; 2021 [acesso 2021 Out 30]. Disponível em: https://bvsms.saude.gov.br/queloide/
6. Kasyanju Carrero LM, Ma WW, Liu HF, Yin XF, Zhou BR. Botulinum toxin type A for the treatment and prevention of hypertrophic scars and keloids: Updated review. J Cosmet Dermatol. 2019;18(1):10-5.
7. Santos CMC, Pimenta CAM, Nobre MRC. The PICO strategy for the research question construction and evidence search. Rev Latino Am Enferm. 2007;15(3):508-11.
8. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467-73.
9. Pruksapong C, Yingtaweesittikul S, Burusapat C. Efficacy of Botulinum Toxin A in Preventing Recurrence Keloids: Double Blinded Randomized Controlled Trial Study: Intraindividual Subject. J Med Assoc Thai. 2017;100(3):280-6.
10. Zhou M, Wang L, Jiang R, Zhu M, Chen F. Evaluation on efficacy and adverse reactions of combined therapy with botulinum toxin type A in treatment of keloid. J Jilin Un (Medicine Ed.). 2017;6:386-90.
11. Rasaii S, Sohrabian N, Gianfaldoni S, Hadibarhaghtalab M, Pazyar N, Bakhshaeekia A, et al. Intralesional triamcinolone alone or in combination with botulinium toxin A is ineffective for the treatment of formed keloid scar: A double blind controlled pilot study. Dermatol Ther. 2019;32(2):e12781.
12. Pires M, Soudo A, Costa MJ. Toxina Botulínica Tipo A no Tratamento das Cicatrizes Hipertróficas por Queimadura em Idade Pediátrica: Caso Clínico. Rev Soc Port Med Fís Reab. 2020;32(3):126-9.
13. Motoki THC, Isoldi FC, Brito MJA, Filho AG, Ferreira LM. Keloid negatively affects body image. Burns. 2019;45(3):610-4.
14. Dai X, Lei TC. Botulinum toxin A promotes the transdifferentiation of primary keloid myofibroblasts into adipocyte-like cells. Basic Clin Pharmacol Toxicol. 2021;129(6):462-9.
15. Lee YI, Kim J, Yang CE, Hong JW, Lee WJ, Lee JH. Combined Therapeutic Strategies for Keloid Treatment. Dermatol Surg. 2019;45(6):802-10.
16. Sohrabi C, Goutos I. The use of botulinum toxin in keloid scar management: a literature review. Scars Burn Heal. 2020;6:2059513120926628.
1. Universidade Estadual do Ceará, Fortaleza,
Ceará, Brazil
2. Santa Casa de Santos, Santos, São Paulo,
Brazil
Corresponding author: Eduardo Lafayette Monteiro Av. Senador Ruy Carneiro, 212, Miramar, João Pessoa, PB, Brazil, Zip Code: 58032-101, E-mail: eduardomlafayette@gmail.com






 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter