

Original Article - Year 2024 - Volume 39 -
Repair of the abdominal wall with acellular bovine pericardial membranes - Part II - Histological and morphometric analyses
Reparação da parede abdominal com membranas acelulares de pericárdio bovino - Parte II - Análises histológicas e morfométricas
ABSTRACT
Introduction: Histological analysis is the main tool for evaluating acellular bioprostheses, mostly on an experimental basis. The objective is to histologically analyze the acellular matrix of bovine pericardium in abdominal wall repairs implanted in humans.
Method: From a series of 30 repairs with the membrane, 3 patients underwent surgical revision unrelated to the implants at 13, 22, and 23 months postoperatively, obtaining biopsies of the previously implanted areas. In addition to evaluating the basic aspects of biocompatibility and tissue neoformation, the slides were digitalized and subjected to computerized analysis with the ImageJ software to quantify the kinetics of membrane degradation associated with the analysis of the fractal dimension of the samples. The values obtained for percentages of residual membrane had their means compared by analysis of variance (ANOVA) and the unpaired Student's T test, also used for the fractal dimension quantification values.
Results: The biocompatibility of the material was demonstrated, with tissue neoformation, collagen deposition, and cellularized tissue with a normal appearance without important local reactions. Residual fragments of the membrane were quantified at 40%±7% at 13 months, at 20%±6% at 22 months, and at 17%±6% at 23 months postoperatively, with the analysis of the fractal dimension indicating a progressive degradation of implants, with statistical significance between 13 months and late samples.
Conclusion: The results confirmed the functionality of the acellular bovine pericardium under different levels of mechanical stress in abdominal wall repairs in humans.
Keywords: Extracellular matrix; Abdominal hernia; Abdominal wall; Prosthetics and implants; Surgical meshes; Bioprosthesis; Pericardium.
RESUMO
Introdução: Análise histológica é a principal ferramenta de avaliação de biopróteses acelulares, em sua maioria em caráter experimental. O objetivo é analisar histologicamente a matriz acelular de pericárdio bovino em reparações de parede abdominal implantada em humanos.
Método: De uma série de 30 reparações com a membrana, 3 pacientes foram submetidas a revisão cirúrgica não relacionada aos implantes, aos 13, 22 e 23 meses de pós-operatório, obtendo-se biópsias das áreas previamente implantadas. Além da avaliação dos aspectos básicos de biocompatibilidade e neoformação tecidual, as lâminas foram digitalizadas e submetidas a análise computadorizada com o software ImageJ para quantificação da cinética de degradação das membranas, associada à análise da dimensão fractal das amostras. Os valores obtidos para porcentagens de membrana residual tiveram suas médias comparadas por análise de variância (ANOVA) e pelo teste T de Student não pareado, também utilizado para os valores da quantificação da dimensão fractal.
Resultados: Foi demonstrada a biocompatibilidade do material, com neoformação tecidual, deposição de colágeno e tecido celularizado de aspecto normal, sem reações locais importantes. Fragmentos residuais da membrana foram quantificados em 40%±7% aos 13 meses, em 20%±6% aos 22 meses e em 17%±6% aos 23 meses de pós-operatório, com a análise da dimensão fractal indicando uma progressiva degradação dos implantes, com significância estatística entre 13 meses e as amostras tardias.
Conclusão: Os resultados atestaram a funcionalidade do pericárdio bovino acelular sob diferentes níveis de estresse mecânico nas reparações da parede abdominal em humanos.
Palavras-chave: Matriz extracelular; Hérnia abdominal; Parede abdominal; Próteses e implantes; Telas cirúrgicas; Bioprótese; Pericárdio
INTRODUCTION
The repair of structural defects with endogenous tissues, undoubtedly a skill of plastic surgeons, is limited in many situations and has stimulated the production of supportive biomaterials, with numerous synthetic materials developed and used on a large scale for applications in various fields of reconstructive surgery.
As part of an evolution of this process, biological prostheses were developed, originating from acellularized natural tissues1, providing biodegradable three-dimensional support for the recipient’s cellular growth and requiring sophisticated degradation kinetics over time2. Basically represented by the extracellular membrane (ECM) resulting from the acellularization process, these membranes develop an active biological role at the implantation site, in theory favoring tissue remodeling rather than the formation of scar fibrosis or chronic inflammation3, concepts pursued in the field of regenerative medicine.
Progressively degraded by metalloproteinases4 - especially collagenase - acellular membranes must support a complex balance between resistance to degradation and promotion of cell growth from the receptor bed, with dynamic reciprocity favoring tissue neoformation and adequate collagen deposition until the repair site has healed adequately. Thus, in addition to the basic aspect of biocompatibility, evaluating the degradation time of the three-dimensional support is also essential, as its very early occurrence can lead to failure of the repair, especially in those that require greater mechanical resistance, such as in the reconstruction of the abdominal wall5.
In this sense, in addition to the differences in relation to their allogeneic or xenogenic origin, as well as their tissue biological nature - dermis, intestinal mucosa, pericardium, etc. - aspects related to the preparation and reticulation processes are described as important factors in the biological behavior of ECMs. Studies demonstrate that reticulation increases the durability of implanted biomaterials, thus providing a greater capacity to provide adequate support for remodeling processes with endogenous collagen in abdominal hernia repairs7.
Numerous publications use histological analyses as the main tool for evaluating these biological processes in different bioprostheses. However, the vast majority are in animal experimentation8,9, with observations in humans restricted to complicated cases of reoperations in the presence of infections and implant removal.10,11
OBJECTIVE
The objective of this publication is to report the histological findings observed in biopsies of acellular bovine pericardial membranes implanted in abdominal wall repair.
METHOD
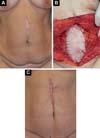
From a series of 40 abdominal wall repairs associated with implantation of acellular bovine pericardial membrane, 3 patients underwent surgical revision, namely 2 cases, secondary to incisional hernias, for correction of hypertrophic scar at 13 months (Figure 1) and 22 months postoperatively, and 1 case, secondary to post-resection reconstruction of wall endometrioma, reviewed at 23 months postoperatively to explore possible recurrence. In all cases, the postoperative evolution was without any complications, with clinical and radiological examinations not identifying problems related to the implanted areas, with successful repairs, with revisions being carried out for indications not related to implants. The patients were duly informed, through a form of consent, that biopsies would be taken in the implant area at the time of eventual surgical revision.
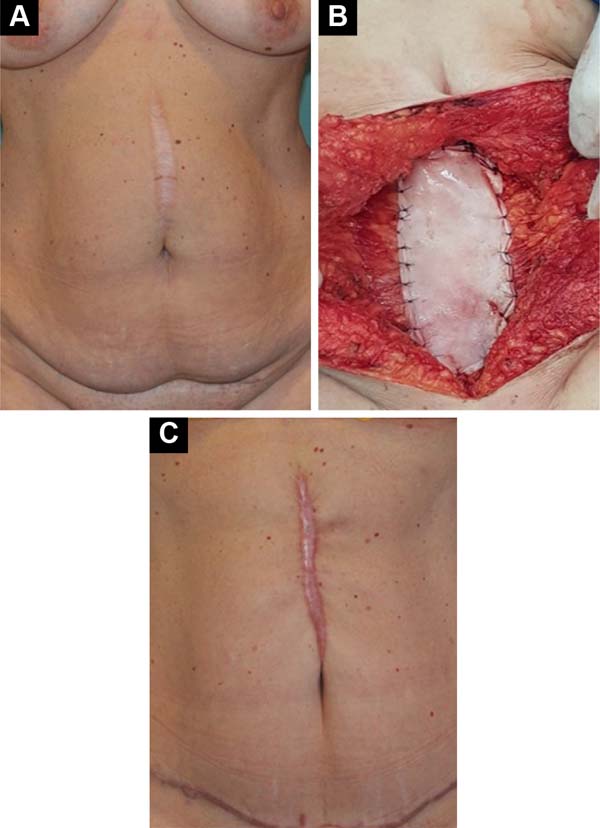
In the areas corresponding to previous implants in a pre-aponeurotic situation, made by the same surgeon and identified photographically, 3 samples were taken at different points in the implanted region, removing samples from the muscular aponeurosis in its entire thickness. After fixation and inclusion in paraffin, serial sections of 5µm thickness were made, with 60 slides being stained for each patient with Hematoxylin - Eosin, Gomori’s Trichrome, and Picrosirius Red for the different analyses.
Morphometric analysis
The slides were examined with a Nikon SI E200 Trinocular optical microscope for the usual stains and with polarized light for Picrosirius Red, and the images were digitized with a Digilab™ jkc camera at 8MB resolution. In addition to the basic aspects regarding the biocompatibility of the material and characteristics of tissue neoformation, aspects of absorption/degradation of the implants and the process of cellularization and collagen deposition in the recipient bed were also analyzed, quantified by computerized analysis using the ImageJ software, specific for this purpose12.
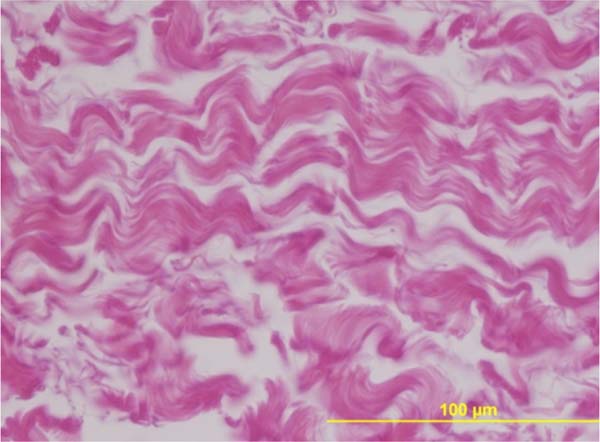
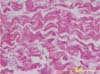
Using the histological image of the “in natura” acellular pericardium as a standard (Figure 2), the percentages of residual membrane present in the different periods were quantified on the HE-stained slides. The acellular pericardium still present in the different samples was identified and delimited manually by two independent examiners, with the corresponding percentage calculated automatically by the software.
On slides stained with Picrosirius Red - specific for collagen fibers - the quantification of tissue fractal dimension was additionally carried out by digital analysis13, also using the ImageJ software, representing tissue fragmentation by a specific automatic method called “Box-Count /Binary - Outline”.
Statistical analysis
The values obtained in the quantification of the percentages of residual membrane had their means statistically compared by analysis of variance (ANOVA) and the unpaired Student’s T test, also used to analyze the values obtained in the quantification of the fractal dimension. An alpha error of 5% was allowed, with p-values less than or equal to 0.05 being considered significant.
RESULTS
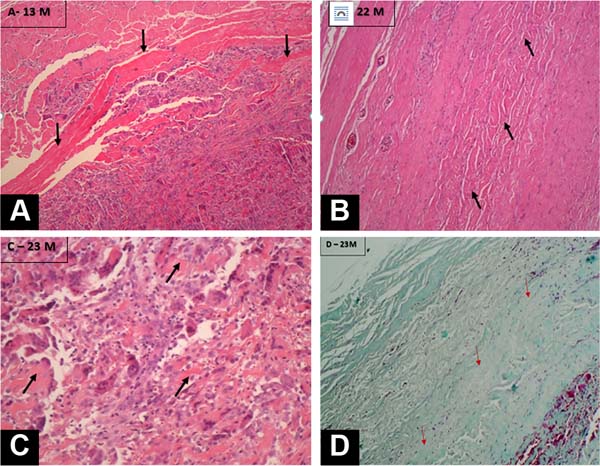
Histological analyses clearly demonstrated the biocompatibility of the material, with all samples showing tissue neoformation replacing the implanted membranes, with significant deposition of collagen and cellularized tissue with a normal appearance. No important local reactions were observed, with some rare isolated focal points being identified showing macrophages in a mild inflammatory process. In all periods analyzed, it was possible to identify the presence of fragments of acellular tissue corresponding to the original membrane (Figure 3).
Using ImageJ software, residual fragments of the implanted membrane were quantified at 40%±7% at 13 months, at 20%±6% at 22 months, and 17%±6% at 23 months postoperatively. This quantification, analyzed by the unpaired t-test, was statistically significant between the 13-month and later samples, with no statistical difference between 22 and 23 months (Graph 1).

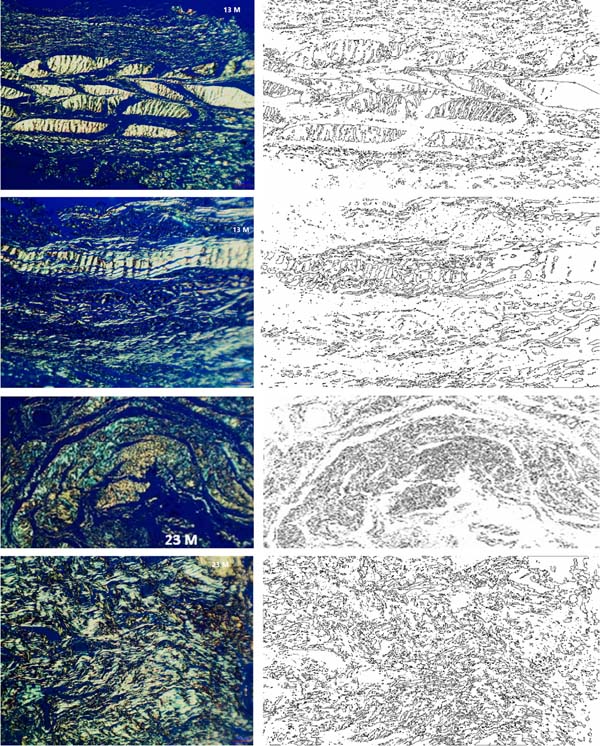
Using Picrosirius staining with polarized light, the fractal dimension of the slides was analyzed at different postoperative periods, also using an automatic method in a specific tool in the ImageJ software, demonstrated in Figure 4.
The distribution of fractal dimension values for each subgroup, using the Box-Plot graph, shows a clear separation of values between the subgroup with the shortest follow-up time (13 months) and the subgroups (together or separately) with 22 and 23 months of follow-up. (Graph 2).

Analysis using the unpaired t-test showed a statistically significant difference between 13 months versus 22 months (p=0.0058), between 13 months versus 23 months (p=0.0128), and between 13 months versus the set of 22 and 23 months (p<0.0001), with an increase in fractal dimension indicating the progressive occurrence of tissue neoformation due to the cellularization process and collagen deposition in the receptor bed. There was no statistically significant difference in the fractal dimension comparing 22 months versus 23 months (p=0.3141).
The two morphometric evaluation methods adopted had concordant findings, with a reduction in the percentage of residual implant demonstrating its progressive absorption/degradation, concomitant with the occurrence of cellularization and collagen deposition evidenced by the progressive increase in the fractal dimension.
DISCUSSION
The exponential increase in the supply of acellular matrices of different origins in recent years and the growth projections of this market14 prove the increasing adoption of bioprostheses in different therapeutic options, as well as in tissue engineering15, as molds for stem cell cultivation16 and in the application of “drug delivery”17, with MECs embedded in medicines with different purposes.
Its differential as an implant in various repair processes lies particularly in its biocompatibility characteristics, the progressive degradation/absorption of the implants, and its concomitant replacement by tissue neoformation. Furthermore, unlike synthetic implants, which can induce a polymer-dependent inflammatory response with the formation of biofilms18,19, acellular bioprostheses exert biological functions “in situ”, favoring regenerative processes20,21, in addition to allowing their application in contaminated and infected surgical sites.22,23
Histological analyses based on experimental models constitute the main tool for evaluating these biological processes, with hundreds of publications describing various aspects of extracellular matrices such as tissue origin, thickness, acellularization methods, reticulation, etc. - in an attempt to indicate the best choices for the different repair processes. In the present study, it was possible to histologically observe the main biological processes in humans under normal conditions, an uncommon condition with aspects not yet described in the literature for abdominal wall repairs.
In the implanted areas, it was possible to observe the incorporation of the pericardial ECM into the recipient bed, with neovascularization and increasing presence of cellularized neotissue and adequate collagen deposition in all periods analyzed, with good quality repair and absence of inflammatory processes or important signs of immune response. In addition to excellent biocompatibility, this demonstrates that the material fulfilled its function as a biological scaffold, favoring the processes of cell adhesion, proliferation, and differentiation, serving as a substrate for tissue repair, a fundamental characteristic expected in biological structures composed of extracellular matrices24.
Similar findings with acellular bioprostheses implanted in humans for breast reconstructions have been reported in the literature, with human25 and porcine dermis26,27, describing the process of integration of ECMs as a form of normal healing, with initial neovascularization followed by progressive cellular repopulation of the matrix with cells of the receptor and absence of foreign body type reactions.
With data also not yet found in the literature, it was possible to quantify the degradation kinetics of the acellular bovine pericardium implanted in the abdominal wall, analyzed by two complementary computerized methods. In all biopsies from areas implanted in different periods, it was possible to identify standard fragments of residual acellular pericardium, which were quantified as a percentage, complemented with the analysis of the fractal dimension of the samples over time.
Both analyses indicated that the process of reabsorption and replacement by neotissue is progressive, with a statistically significant difference, observing that around 60% of the implant was reabsorbed after 13 months post-surgery and around 80% after around two years, suggesting that the entire matrix should be degraded in the long term.
Other publications also describe the degradation kinetics in percentages of residual or absorbed ECM for porcine dermis and intestinal serosa, also with morphometry computerized, by multispectral analysis of histological images28 or with matrices marked with Carbon-1429. The results show the presence of residual membrane for up to 90 days for non-reticulated intestinal serous matrices, disappearing around 180 days and, for reticulated dermal matrices, much slower reabsorption, with the presence of 80% of the implant in the first 4 weeks and 50 % still present at around 6 months.
As described in the literature7,8,30, this aspect confirms the greater resistance to degradation of the reticulated matrix used and may represent an advantage for repairs in which greater long-term mechanical resistance is required, such as in the abdominal wall. The functionality of degradable materials depends on the balance between the rate of degradation and the rate of tissue remodeling in the host bed, and it is necessary to understand not only the biological response to degradable biomaterials but also the expected mechanical properties of the implant and replacement tissues over time for each therapeutic application31.
These findings are compatible with several clinical and experimental studies using different ECMs in abdominal wall repairs32,33, also including bovine pericardium34, showing very satisfactory characteristics for their use even in high-risk situations35. In a comparative analysis with the vast literature presented, the results highlight the translational nature of the experimental models used to evaluate and characterize acellular matrices and demonstrate the close similarity of the pericardium used with those general characteristics and therapeutic applications. However, numerous particular variables can affect clinical results36-38, highlighting here for discussion specific aspects of the receptor bed itself and the matrix used in terms of acellularization, reticulation and its presentation in liquid media.
The action of biomechanical forces acting in different locations can differentially affect collagen distribution and tissue remodeling of biological molds39, which is a fundamental component to be considered when using ECMs in the abdominal wall40. The results obtained demonstrated good-quality tissue neoformation in all samples, attesting to the functionality of the implant under different levels of mechanical stress on the abdominal wall.
The pericardium used is fixed in glutaraldehyde - a technique used effectively for decades in acellular matrices41 - and soaked post-fixation in 4% formaldehyde and is sold in this way. In addition to glutaraldehyde promoting a reduction in connective tissue antigenicity and stabilization against chemical and enzymatic degradation in varying degrees of “reticulation” 42,43, this association has well-described terminal sterilization effects44. This important factor can also affect the structural properties of acellular matrices45. In addition to simpler processing, maintenance in liquid media is described as advantageous for tissue architecture, avoiding collapse and preserving matrix components that provide mechanical and biochemical benefits after implantation46.
Although freeze-drying facilitates the manipulation and long-term preservation of ECMs, factors can affect their performance both during their synthesis, with disturbances of collagen fibers47, and at the time of their implantation, with rehydration time being able to alter their biomechanical and physical properties significantly. -chemicals48. We can speculate that these factors also favored the behavior of the membrane used, both due to its biocompatibility and its observed degradation kinetics.
CONCLUSION
Histological analyses demonstrated similarity with all the biological characteristics described in the literature for acellular tissue matrices, and the process of integration and incorporation of ECMs could be observed in the samples, with neovascularization followed by progressive cellular repopulation of the matrix with receptor cells and collagen deposition with good healing quality, demonstrated by the increase in fractal dimension. Also relevant in humans, the degradation kinetics of the bovine pericardium matrix was quantified at approximately 60% after 13 months and 80% after approximately two years, suggesting that the entire matrix may be degraded over a longer period.
Under both aspects, the results attested to the functionality of the acellular bovine pericardium under different levels of mechanical stress in abdominal wall repairs in humans.
REFERENCES
1. Baumann DP, Butler CE. Bioprosthetic mesh in abdominal wall reconstruction. Semin Plast Surg. 2012;26(1):18-24.
2. Panayi AC, Orgill DP. Current Use of Biological Scaffolds in Plastic Surgery. Plast Reconstr Surg. 2019;143(1):209-20. DOI: 10.1097/PRS.0000000000005102
3. Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014;163(4):268-85.
4. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516.
5. Blatnik J, Jin J, Rosen M. Abdominal hernia repair with bridging acellular dermal matrix--an expensive hernia sac. Am J Surg. 2008;196(1):47-50.
6. Costa A, Naranjo JD, Londono R, Badylak SF. Biologic Scaffolds. Cold Spring Harb Perspect Med. 2017;7(9):a025676. DOI: 10.1101/cshperspect.a025676
7. Smart NJ, Bloor S. Durability of biologic implants for use in hernia repair: a review. Surg Innov. 2012;19(3):221-9.
8. Liang HC, Chang Y, Hsu CK, Lee MH, Sung HW. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials. 2004;25(17):3541-52.
9. Mestak O, Spurkova Z, Benkova K, Vesely P, Hromadkova V, Miletin J, et al. Comparison of Cross-linked and Non-Cross-linked Acellular Porcine Dermal Scaffolds for Long-term Full-Thickness Hernia Repair in a Small Animal Model. Eplasty. 2014;14:e22.
10. Wotton FT, Akoh JA. Rejection of Permacol mesh used in abdominal wall repair: a case report. World J Gastroenterol. 2009;15(34):4331-3.
11. Cheung D, Brown L, Sampath R. Localized inferior orbital fibrosis associated with porcine dermal collagen xenograft orbital floor implant. Ophthalmic Plast Reconstr Surg. 2004;20(3):257-9.
12. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-5.
13. Backes AR, Bruno OM. Técnicas de estimativa de dimensão fractal aplicadas em imagens digitais. Relatórios Técnicos. São Carlos: Universidade de São Paulo; 2005. Disponível em: http://repositorio.icmc.usp.br//handle/RIICMC/6846
14. Acellular Matrix Treatment Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2021 - 2031. Disponível em: https://www.transparencymarketresearch.com/acellular-dermal-matrix-treatment-market.html
15. Knight RL, Wilcox HE, Korossis SA, Fisher J, Ingham E. The use of acellular matrices for the tissue engineering of cardiac valves. Proc Inst Mech Eng H. 2008;222(1):129-43. DOI: 10.1243/09544119JEIM230.
16. Iyyanki TS, Dunne LW, Zhang Q, Hubenak J, Turza KC, Butler CE. Adipose-derived stem-cell-seeded non-cross-linked porcine acellular dermal matrix increases cellular infiltration, vascular infiltration, and mechanical strength of ventral hernia repairs. Tissue Eng Part A. 2015;21(3-4):475-85. DOI: 10.1089/ten.tea.2014.0235
17. Friess W. Collagen--biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45(2):113-36.
18. Robinson TN, Clarke JH, Schoen J, Walsh MD. Major mesh-related complications following hernia repair: events reported to the Food and Drug Administration. Surg Endosc. 2005;19(12):1556-60.
19. Klosterhalfen B, Klinge U, Hermanns B, Schumpelick V. Pathology of traditional surgical nets for hernia repair after long-term implantation in humans. Chirurg. 2000;71(1):43-51. German.
20. Melman L, Jenkins ED, Hamilton NA, Bender LC, Brodt MD, Deeken CR, et al. Early biocompatibility of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral hernia repair. Hernia. 2011;15(2):157-64. DOI: 10.1007/s10029-010-0770-0
21. Connor J, McQuillan D, Sandor M, Wan H, Lombardi J, Bachrach N, et al. Retention of structural and biochemical integrity in a biological mesh supports tissue remodeling in a primate abdominal wall model. Regen Med. 2009;4(2):185-95. DOI: 10.2217/17460751.4.2.185
22. Brennan EP, Reing J, Chew D, Myers-Irvin JM, Young EJ, Badylak SF. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006;12(10):2949-55.
23. Harth KC, Broome AM, Jacobs MR, Blatnik JA, Zeinali F, Bajaksouzian S, et al. Bacterial clearance of biologic grafts used in hernia repair: an experimental study. Surg Endosc. 2011;25(7):2224-9.
24. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5(1):1-13.
25. Boháč M, Danišovič Ľ, Koller J, Dragúňová J, Varga I. What happens to an acellular dermal matrix after implantation in the human body? A histological and electron microscopic study. Eur J Histochem. 2018;62(1):2873. DOI: 10.4081/ejh.2018.2873
26. Katerinaki E, Zanetto U, Sterne GD. Histological appearance of Strattice tissue matrix used in breast reconstruction. J Plast Reconstr Aesthet Surg. 2010;63(12):e840-1. DOI: 10.1016/j.bjps.2010.06.033
27. Salzberg CA, Dunavant C, Nocera N. Immediate breast reconstruction using porcine acellular dermal matrix (Strattice™): long-term outcomes and complications. J Plast Reconstr Aesthet Surg. 2013;66(3):323-8. DOI: 10.1016/j.bjps.2012.10.015
28. Costa A, Naranjo JD, Turner NJ, Swinehart IT, Kolich BD, Shaffiey SA, et al. Mechanical strength vs. degradation of a biologically-derived surgical mesh over time in a rodent full thickness abdominal wall defect. Biomaterials. 2016;108:81-90.
29. Carey LE, Dearth CL, Johnson SA, Londono R, Medberry CJ, Daly KA, et al. In vivo degradation of 14C-labeled porcine dermis biologic scaffold. Biomaterials. 2014;35(29):8297-304. DOI: 10.1016/j.biomaterials.2014.06.015
30. de Castro Brás LE, Shurey S, Sibbons PD. Evaluation of crosslinked and non-crosslinked biologic prostheses for abdominal hernia repair. Hernia. 2012;16(1):77-89. DOI: 10.1007/s10029-011-0859-0
31. Badylak S, Kokini K, Tullius B, Whitson B. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res. 2001;99(2):282-7. DOI: 10.1006/jsre.2001.6176
32. López Cano M, Armengol Carrasco M, Quiles Pérez MT, Arbós Vía MA. Biological implants in abdominal wall hernia surgery. Cir Esp. 2013;91(4):217-23.
33. Lotan AM, Cohen D, Nahmany G, Heller L, Babai P, Freier-Dror Y, et al. Histopathological Study of Meshed Versus Solid Sheet Acellular Dermal Matrices in a Porcine Model. Ann Plast Surg. 2018;81(5):609-14.
34. Limpert JN, Desai AR, Kumpf AL, Fallucco MA, Aridge DL. Repair of abdominal wall defects with bovine pericardium. Am J Surg. 2009;198(5):e60-5.
35. Shieh MK. Bovine Pericardium in Complex Abdominal Wall Reconstruction in Patients with Obesity or Morbid Obesity. Bariatric Times. 2014;11(9):14-8.
36. Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33(6):1771-81.
37. Tierney CM, Haugh MG, Liedl J, Mulcahy F, Hayes B, O’Brien FJ. The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J Mech Behav Biomed Mater. 2009;2(2):202-9.
38. Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng. 2014;42(7):1517-27.
39. Cavallo JA, Roma AA, Jasielec MS, Ousley J, Creamer J, Pichert MD, et al. Remodeling characteristics and collagen distribution in biological scaffold materials explanted from human subjects after abdominal soft tissue reconstruction: an analysis of scaffold remodeling characteristics by patient risk factors and surgical site classifications. Ann Surg. 2015;261(2):405-15. DOI: 10.1097/SLA.0000000000000471
40. Ventral Hernia Working Group; Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544-58. DOI: 10.1016/j.surg.2010.01.008
41. Reece IJ, van Noort R, Martin TR, Black MM. The physical properties of bovine pericardium: a study of the effects of stretching during chemical treatment in glutaraldehyde. Ann Thorac Surg. 1982;33(5):480-5.
42. Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21(22):2215-31. DOI: 10.1016/s0142-9612(00)00148-4
43. Jayakrishnan A, Jameela SR. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials. 1996;17(5):471-84.
44. Gorman SP, Scott EM, Russell AD. Antimicrobial activity, uses and mechanism of action of glutaraldehyde. J Appl Bacteriol. 1980;48(2):161-90.
45. Freytes DO, Stoner RM, Badylak SF. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84(2):408-14. DOI: 10.1002/jbm.b.30885
46. Faleris JA, Hernandez RM, Wetzel D, Dodds R, Greenspan DC. In-vivo and in-vitro histological evaluation of two commercially available acellular dermal matrices. Hernia. 2011;15(2):147-56. DOI: 10.1007/s10029-010-0749-x
47. Freytes DO, Tullius RS, Valentin JE, Stewart-Akers AM, Badylak SF. Hydrated versus lyophilized forms of porcine extracellular matrix derived from the urinary bladder. J Biomed Mater Res A. 2008;87(4):862-72. DOI: 10.1002/jbm.a.31821
48. Bottino MC, Jose MV, Thomas V, Dean DR, Janowski GM. Freeze-dried acellular dermal matrix graft: effects of rehydration on physical, chemical, and mechanical properties. Dent Mater. 2009;25(9):1109-15. DOI: 10.1016/j.dental.2009.03.007
1. Hospital do Coração de São José do Rio Preto,
São José do Rio Preto, SP, Brazil
2. Instituto Frascino, Unidade de Publicação e
Pesquisa, São José do Rio Preto, SP, Brazil
3. Faculdade de Ciências Médicas da Santa Casa de
São Paulo, São Paulo, SP, Brazil
4. Faculdade de Medicina de São José do Rio Preto,
São José do Rio Preto, SP, Brazil
Corresponding author: Luiz Fernando Frascino Av. Juscelino Kubitscheck de Oliveira, 3700, São José do Rio Preto, SP, Brazil, Zip Code: 15093-225, E-mail: drfrascino@gmail.com
Article received: March 14, 2023.
Article accepted: December 5, 2023.
Conflicts of interest: none.







 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter