

Original Article - Year 2024 - Volume 39 -
Abdominal wall repair with acellular bovine pericardial membranes - Part I - Clinical and radiological findings
Reparação da parede abdominal com membranas acelulares de pericárdio bovino - Parte I - Achados clínicos e radiológicos
ABSTRACT
Introduction: Reducing recurrence rates significantly, the use of biomaterials as "reinforcement meshes" in the repair of different abdominal wall defects has become an almost mandatory routine for the success of these repairs. From the 1990s onwards, acellular biological matrices were introduced, thus beginning a new era in the repair of abdominal wall defects. The objective is to evaluate the functionality of the acellularized bovine pericardium in abdominal wall repairs.
Method: Thirty patients underwent repair of abdominal wall defects using acellular bovine pericardium bioprostheses, making a total of 40 anatomically individualized implants. The average follow-up was 31 months, with patients being evaluated clinically and radiologically. In three cases, biopsies were taken from the implanted areas, allowing histological analysis of the material.
Results: No recurrence of herniations was observed in any of the cases, both clinically and radiologically. There were also no records of bruises, infections or any phenomenon of a local or systemic reaction nature. Radiologically, it was not possible to visualize the matrices at the implantation site in any of the postoperative periods analyzed.
Conclusion: The matrices showed similarity to other biological membranes described in the international literature. Representing an important update and conceptual evolution, acellular bovine pericardial membranes can be incorporated into the therapeutic arsenal in abdominal wall repairs.
Keywords: Prostheses and implants; Hernia, abdominal; Surgical mesh; Extracellular matrix; Abdominal wall; Bioprosthesis; Pericardium.
RESUMO
Introdução: Reduzindo os índices de recidiva de forma impactante, o emprego de biomateriais como "telas de reforço" na reparação de diferentes defeitos da parede abdominal tornou-se rotina quase obrigatória para o sucesso dessas reparações. A partir da década de 1990 houve a introdução de matrizes biológicas acelulares, iniciando-se assim uma nova era na reparação dos defeitos da parede abdominal. O objetivo é avaliar a funcionalidade do pericárdio bovino acelularizado em reparações da parede abdominal.
Método: Trinta pacientes foram submetidos a reparação de defeitos da parede abdominal, com biopróteses acelulares de pericárdio bovino, perfazendo um total de 40 implantes anatomicamente individualizados. O seguimento médio foi de 31 meses, sendo os pacientes avaliados clinicamente e radiologicamente. Em três casos foram feitas biópsias das áreas implantadas permitindo análise histológica do material.
Resultados: Não se observou recidiva das herniações em nenhum dos casos, tanto clinica como radiologicamente. Também não houve registro de hematomas, infecções ou qualquer fenômeno de natureza reacional local ou sistêmica. Radiologicamente, não foi possível visualizar as matrizes no local de implantação em qualquer dos períodos de pós-operatório analisados.
Conclusão: As matrizes mostraram similaridade às demais membranas biológicas descritas na literatura internacional. Representando uma importante atualização e evolução conceitual, as membranas acelulares de pericárdio bovino podem ser incorporadas ao arsenal terapêutico nas reparações de parede abdominal.
Palavras-chave: Próteses e implantes; Hérnia abdominal; Telas cirúrgicas; Matriz extracelular; Parede abdominal; Bioprótese; Pericárdio
INTRODUCTION
Compromising the anatomical and functional integrity of the wall musculoaponeurosis of the abdomen is a relatively frequent occurrence, with varying degrees of clinical manifestation, complexity, and causal agent, being represented mainly by hernias in their different forms by laxity or exaggerated compliance of the anterior abdominal plane secondary to obesity or pregnancy and by post-infection sequelae, trauma or tumor resections.
Data from the public network in Brazil show an annual average of 242 thousand herniorrhaphies, with 54% corresponding to inguinal hernias, 99.4% being open, and only 0.6% laparoscopic, with 22% reported as operated in an emergency situation1. Added to this are patients operated on in private networks and repairs secondary to other causes, indicating the magnitude of the problem and requiring an algorithm in the appropriate selection of the technique to be adopted in the different corrections2.
Furthermore, diffuse bulging due to laxity of the entire abdominal wall, with functional and/or aesthetic impairment, is also a common occurrence and not properly estimated in post-bariatric surgery patients, in pregnancy of repetition, or women with an android biotype, affecting to varying degrees the quality of life and work capabilities in a much larger portion of the population, with great demand for plastic surgeons for treatment. Although complete in their integrity, these repairs generally require reinforcement with mesh after plication of the muscular wall due to the thinning of the structures, preventing a recurrence of protrusion with the functional and cosmetic compromise of the result3.
Although comorbidity factors such as obesity, diabetes, and smoking can affect recurrence rates4, the association of biomaterials made it possible to repair different abdominal wall defects with significant tension relief concomitant with increased local resistance, reducing these rates by more than 50% and demonstrating the mechanical factor as preponderant in the success of repairs5, making the use of “reinforcement screens” mandatory in these situations.
After decades of using synthetic meshes6, from the 1990s onwards, there was the introduction of biological membranes of animal or human origin - called extracellular matrices (ECMs) - thus beginning a new era in the repair of abdominal wall defects7, with results that stimulated its increasing adoption in various parts of the world over the last two decades.
Different types of acellularized membranes have been developed from varied biological tissues - such as human8 or animal dermis9, intestinal mucosa10, bovine fetal tissue11, and bovine pericardium12 - each with relatively distinct characteristics in terms of clinical and therapeutic applications, described in countless scientific publications, generally establishing a new standard of indications in the repair of abdominal wall defects, as well as in several other areas (Table 1).
| Species of Origin | Origin Fabric | Application | Name/Manufacturer |
|---|---|---|---|
| Human | Dermis | Soft Parts /
Breast Soft Parts / Breast Breast/Tendon Breast Soft Parts Pelvic Organ Prolapse Chronic Wounds |
AlloDerm /
Lifecell AlloMax / Bard Davol AlloPatch / Musculosk Foundation Neoform / Mentor Worldwide GraftJacket / Kinetc Concepts Axis / Coloplast DermaPure / Regenix Group |
| Fascia Lata | Ophthalmology Pelvic Organ Prolapse |
Tutoplast
FL/IOP Suspend / Coloplast |
|
| Pericardium | Ophthalmology | IO Patch / IOP | |
| Bovine | Dermis | Soft Parts | TissueMend / Stryker |
| Pericardium | Breast, Vascular, Dura mater, Fascia Dentistry Dura mater Heart Valve |
Veritas, Dura-Guard,
PeriGuard, Vascu-Guard / BAXTER CopiOs / Zimmer Lyoplant / B. Braun Melsungen Perimount / Edwards Lifesciences |
|
| Porcine | Dermis | Soft
Parts/Fascia/Breasts Soft Parts Soft Parts, Breasts, Fascia |
Strattice /
LifeCell ColaMend, XenMatrix / Bard Davol Permacol / Tissue Science Lab |
| Intestinal mucosa | Breasts,
fascia, Dura mater Nerve Repair Pericardium / Cardiac Tissue |
Surgisis,
Durasis, Oasis / Cook Biotech AxoGuard / AxoGen CorMatrixECM / CorMatrix Cardiov |
|
| Urinary bladder | Soft Parts | MatriStem/ACell | |
| Equine | Pericardium | Soft Parts /Chronic Wounds | Unite / Synovis Ort. Wound Care |
| Dura mater | DurAdapt / Pegasus Biologic |
Using EMCs produced by a company with expertise in the production of bovine pericardium bioprostheses for 40 years13, this protocol analyzed the application of biological membranes - called Periwall® - in abdominal wall repair surgery, with aspects not yet described in humans under normal conditions and no publications on the subject in the national scientific literature were identified.
OBJECTIVE
The main objective of the study was to observe the functionality of acellular bovine pericardial membranes in repairing the abdominal wall, as well as evaluate their similarity with other bioprostheses described in the literature.
METHOD
From April 2018 to January 2022, thirty patients underwent abdominal wall repair using the acellular bovine pericardium membrane, 14 men and 16 women, aged between 29 and 77 years (average=48 years), for different indications and locations, making a total of 40 anatomically individualized implants, summarized in Table 2. The minimum follow-up was 10 months, and the maximum was 46 months, with an average of 31 months.
| Diagnosis | Number of Cases | Number of Implants |
|---|---|---|
| Hernias | ||
| Unilateral Inguinal | 10 | 10 |
| Bilateral Inguinal | 03 | 06 |
| Incisional | 04 | 04 |
| Umbilical/Paraumbilical | 02 | 02 |
| Epigastric | 01 | 01 |
| Post Bariatric / Abdominal Wall Compliance | 06 | 09 |
| Infraumbilical Wall Endometrioma | 01 | 01 |
| Associated Deformities | ||
| Inguinal Hernia + Supra Umbilical Hernia | 01 | 02 |
| Post Bariatric + Supra Umbilical Hernia | 01 | 02 |
| Wall Compliance + Bilateral Inguinal Hernia | 01 | 03 |
| Total | 30 | 40 |
On a multicenter basis and duly approved by CONEP and the Research Ethics Committees, patients were selected and operated on jointly by the main author and general surgery teams with expertise in the area at three reference hospitals in São José do Rio Preto - Beneficência Portuguesa, Hospital do Coração and Hospital Estadual João Paulo II. All patients received an informed consent form about the nature of the procedure, describing the mandatory need for reinforcing mesh to correct the problem and the options available between the synthetic and biological compounds.
In addition to the main pathology and the classification of the nature of the surgical wounds, the variables age, Body Mass Index, and any associated pathologies, important for the study’s inclusion/exclusion criteria, were recorded. Only two inclusion criteria were used, namely - 1. Only surgeries classified as “Clean” were admitted and 2. In cases where the indication corresponded to the conventional standard described in the literature for the use of synthetic meshes, that is, in surgeries in which the implantation of abdominal wall reinforcement was mandatory. Pre-operatively, obese patients, those with contaminated or infected surgical wounds, and those with important associated comorbidities (high blood pressure, type 1 diabetes, emphysema, and/or obstructive pulmonary disease) were excluded, with a risk-benefit ratio considered unfavorable by the medical team.
All patients underwent surgery under general anesthesia, with an average hospital stay of 1 day, and received antibiotic therapy with cephalosporin 1g per day for 7 days. The use of a compressive abdominal belt was recommended for 120 days, as well as the prohibition of strenuous physical activities during this period.
In addition to monthly clinical monitoring, radiological ultrasound examinations were performed at different postoperative periods and, in 4 cases, electromagnetic resonance imaging of the abdominal wall at 9, 11, 17, and 26 months.
In 3 patients, it was possible to take biopsies of the implanted areas at 13, 22, and 23 months postoperatively. The slides were stained with hematoxylin-eosin, Gomori’s trichrome, and Picro-Sírius Red and subjected to morphometric analysis.
Membrane preparation
The membranes were supplied sterile in vials containing 4% formaldehyde, requiring pre-implantation washing, as recommended by the manufacturer. Thus, the membrane was placed in a vat containing 0.9% saline solution and manually shaken, discarding the solution every 5 minutes and repeating the procedure for 3 cycles, then requiring 15 minutes for the material to be in terms of use. The “no touch” principles were followed when handling the implants and preparing the store, with changing and washing gloves to remove talc residue and re-antisepsis of the surgical site prior to implantation, thus favoring minimal manipulation. A solution was prepared containing 1000ml of 0.9% saline solution associated with 2g of cephalosporin and 80mg of gentamicin, leaving the already washed membrane immersed in this solution until use. During the fixation process, the same solution was used to irrigate the entire implantation area at random periods.
Surgical technique
The membranes were fixed with separate and/or continuous points of non-absorbable polypropylene (Prolene®) 2-0 or 3-0 threads on their periphery, complemented with multiple “mattress pad” adhesion points on their surface, avoiding folds of the implant. Routinely, absorbable sutures made of polyglycolic acid 2/0 (Vicryl®) or Polydioxanone (PDS®) 2/0 were applied subcutaneously to the membranes and muscular aponeurosis along the entire length of the detached area for immobilization of the flap and maximum reduction of dead spaces, preventing seromas and promoting the largest possible contact surface of the membrane-tissue interface. No suction drains were used in any of the cases in this series.
In cases of inguinal hernias (19 cases), the reinforcement membrane was fixed to the inguinal canal with 2/0 Prolene stitches and kept buried in a subfascial position (Figure 1A). When closing the aponeurosis, 2/0 Vicryl® stitches were applied, plicating the membrane as described. The average size of the implants was 10 x 6cm.
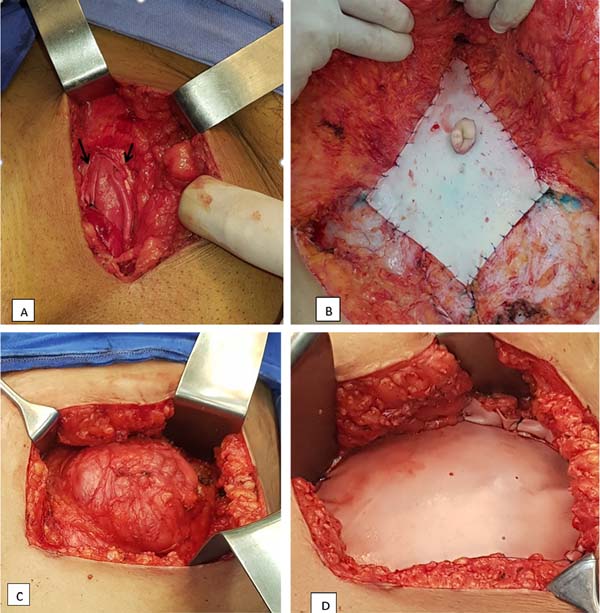
In umbilical and epigastric hernias (5 cases), it was possible to make a direct primary approximation of the muscles and aponeurosis, with separate sutures of 1/0 poliglecaprone (Caprofyl®), with the membrane affixed in a suprafascial position, sutured, covering the treated area with separate threads. of Prolene 3/0 (Figure 1B). The average size of the implants was 6 x 6cm.
In 3 incisional hernias, the same procedure was used, with the membranes in a suprafascial position and an average size of 10 x 10cm. In one case, primary approximation of the muscles was not possible, with the membrane being exceptionally positioned in a bridge, directly sutured under tension externally to the edges of the hernial ring and over the peritoneum (Figures 1C and D).
In repairs due to exaggerated compliance of the wall (8 cases) and post-tumor resection reconstruction (1 case), after horizontal and vertical muscle plication with 1-0 absorbable threads of Caprofyl® or polydioxanone (PDS®), the membranes were sutured in position suprafascial with 3/0 Prolene stitches. These cases required the largest membranes - 15 x 10cm - as well as the need to use more than one membrane, with supra and infraumbilical positioning in 3 cases.
RESULTS
Clinical evaluations
The patients presented good results, with no recurrence of herniations in any of the cases, both clinically and radiologically. There were also no records of bruises, infections or any phenomenon of a local or systemic reaction nature. In 1 case, the patient presented infraumbilical seroma, treated by needle aspiration with the removal of 60ml of secretion, without other recurrences.
Three patients underwent late reoperation, 2 for scar revision at 13 and 22 months postoperatively, and 1 for post-endometrioma resection revision at 23 months. In all cases, it was not possible to visually identify the previously implanted membrane, which was then incorporated into the recipient bed, with a normal appearance, without reactional areas, and with slight surgical fibrosis in the region. In all cases, biopsies were taken in the region corresponding to the previous implantation of the membrane.
Radiological evaluations
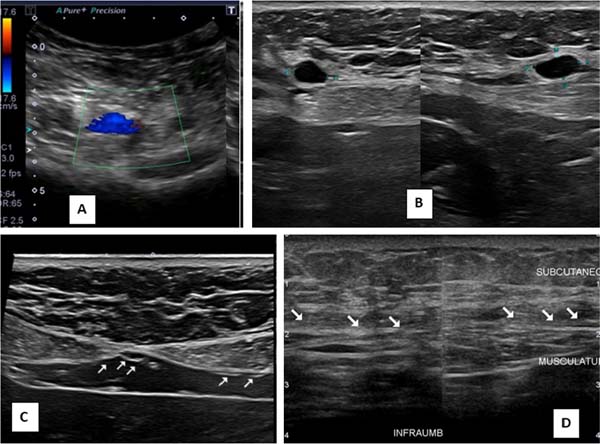
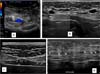
Ultrasounds carried out at different periods did not allow any identification of the implanted membranes, even early on. In the first 30 days after surgery, small, scattered seromas could be observed in the area of the implants, with no clinical relevance, disappearing after this period. No long-term local recurrences were observed, nor were there any anatomical changes in the operated region (Figures 2A-D).
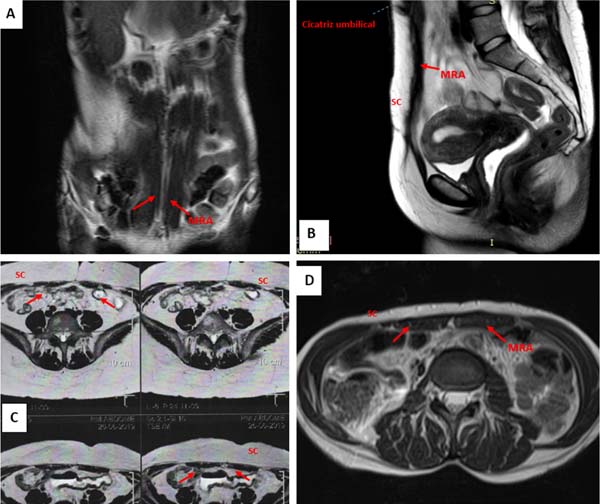
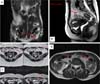
In electromagnetic resonances, carried out at 9, 11, 17, and 26 months postoperatively, it was also not possible to identify the membranes in the implantation region, as well as indirect signs of their presence. No local recurrences or important anatomical changes in the operated areas were identified (Figures 3A-D).
Histological evaluations
In all samples, biopsies showed tissue neoformation replacing the implanted membranes, with significant deposition of collagen and normal-looking cellularized tissue, with no important reactional aspects observed. The findings and morphometric and statistical analyses are described in detail in a parallel publication to this one (part II).
DISCUSSION
Acellular biological matrices have been increasingly used not only in the reconstruction of the abdominal wall but also in various therapeutic applications, revealing a conceptual evolution in the application of biomaterials. The nature of the three-dimensional molecular organization distinguishes biological extracellular matrix templates from synthetic materials due to the possibility of repair through tissue remodeling instead of scar fibrosis, strategies pursued by the concepts of tissue engineering and regenerative medicine14.
Bovine pericardium has been successfully adopted for this purpose, with a relative advantage over synthetic materials due to its ability to be incorporated into surrounding tissues, demonstrating resistance to infection, extrusion, erosion, and formation of visceral adhesions15-17. Acellular membranes have been particularly indicated in the presence of contaminated wounds and situations of direct contact between the implant and the viscera18,19.
The results observed allow us to state that the pericardium matrix used had similar behavior to that documented in the literature, presenting functionality in correcting defects without recording recurrences or reactional events and integration with adjacent tissues, with the neotissue offering mechanical resistance satisfactory in the long term. It was possible to directly observe the total incorporation of the membranes during reoperations, in addition to the results described in the radiological evaluations. In this aspect, although attesting to the anatomical integrity of the operated sites, the matrices were not visualized by ultrasound from 15 days after surgery, as well as by electromagnetic resonance from 9 months onwards, thus revealing the ineffectiveness of the methods as a tool for evaluation of ECMs in the postoperative period.
The protocol adopted did not allow for a comparative evaluation of the membranes in more complex repairs and in contaminated wounds. However, due to the similarity presented with the general characteristics of ECMs described in the available literature, their indication can be considered without restrictions in future repairs of the abdominal wall in those conditions. Deliberately, the protocol limited the indications to lower-risk cases, classified as Grade I20, limiting comorbidities that could interfere with the evaluation of the implant itself in the event of complications.
It is estimated that up to 75% of complications are due to infection, seromas, and inadequate implant fixation21,22, justifying the preventive methodology adopted. The routine prevention of biofilm formation23, a common cause of adverse events in the use of implants, justifies the rigorous adoption of the “no touch” concept intraoperatively24,25. The same applies to the prevention of seromas and implant fixation, with “subcutaneous/implant/aponeurosis” adhesion points efficiently fulfilling several objectives.
With the immediate immobilization of the flap and implant, slippage is prevented, and the greatest possible contact of the membrane/tissue interface is promoted, favoring the biological processes of membrane repair and incorporation. Furthermore, the presence of “dead space” in the detachment plane is reduced as much as possible, preventing seromas and simultaneously eliminating the need for drains.
No aspiration drains were used in any of the cases in this series, and in only one case, there was a need for aspiration puncture to treat a small seroma. Ultrasounds performed at different postoperative periods show small, scattered seromas present in the first 30 days, not being observed in late cases, with a normal-looking subcutaneous/aponeurosis interface without visualization of the membranes.
Besides being more comfortable for the patient the absence of drains, its use may favor contamination of the surgical site, and its effectiveness in preventing seromas has not been adequately demonstrated26,27, with use restricted to exceptional indications. The results obtained confirm that rigorous fixation of the subcutaneous/aponeurosis/implant interface, with progressive adhesion sutures of the entire detached area28, can eliminate the need for aspiration drains in abdominal wall repairs in the presence of implants.
Technical aspects in the placement and positioning of implants in relation to the muscular plane - subfascial, suprafascial, submuscular, or “bridged” - are factors described that can also increase the risk of complications29,30. In the cases operated on in this series, the membranes were implanted in the subfascial space for inguinal and suprafascial hernias in protrusions and repairs of incisional hernias with primary approximation of the muscles, being kept in bridge exceptionally in only 1 case. Aided by fixation methods, the results show that suprafascial fixation of bioprostheses is a simple and effective method for correcting less complex ventral hernias, as described in other publications31, as well as in common cases due to exaggerated compliance of the abdominal wall (Video 1ttp://rbcp.org.br/Content/imagebank/video/REPARA%C3%87%C3%83O DA PAREDE ABDOMINAL.mp4).
Biological prostheses must be biodegraded by the action of metalloproteases32 and replaced by native tissues over time, serving as a temporary structure for the growth of host cells. If the implant is absorbed before adequate processes of neovascularization, tissue growth, and collagen differentiation/deposition, the quality of the neotissue will compromise the expected result, a concept that determines the functionality of the implants.
In this context, discussions focus on whether or not to cross-link ECMs for abdominal wall repairs33,34 due to different biological behaviors. Non-cross-linked matrices present faster incorporation and reabsorption35, while cross-linking prolongs degradation time and increases mechanical resistance36,37.
Studies have shown that EMCs cross-linked with glutaraldehyde have greater resistance to collagenase, with slower degradation, particularly in contaminated environments38,39, which is why they have been the first choice to be considered in these situations for more than a decade21,40. Studies with acellularized bovine pericardium demonstrated a direct relationship between the degree of cross-linking and resistance to degradation, which is decisive in the degradation kinetics and the pattern of tissue regeneration41,42, making it seem logical to adopt cross-linked bioprostheses in regions of greater mechanical stress, such as the abdominal wall.
The glutaraldehyde cross-linked matrix used in this study showed similar properties to other bioprostheses reported in terms of progressive material degradation requirements versus mechanical resistance of the newly formed tissue. In addition to the clinical and radiological findings, direct visualization of the reoperated sites showed the intact wall with good quality scar tissue, without adhesions or important inflammatory processes, with the implanted membranes fully incorporated.
Extracellular matrices represent a microenvironmental niche with important biological activities in tissue regeneration processes43, going beyond the merely structural issue of mechanical support. With implantation, processes begin that impact local biological activity with cellular recruitment and immune response44-46, favoring an environment of functional remodeling rather than scarring fibrosis or chronic inflammatory processes.
Unlike the exclusively mechanical role of synthetic implants, bioprostheses play an active role in in situ biological events, pointing towards regenerative processes that must be properly known and experienced by surgeons, whose experience is estimated as one of the most important prognostic factors in the correction of abdominal wall hernias47.
CONCLUSION
The acellular bovine pericardium matrix was effective in correcting abdominal wall defects, attesting to its functionality and similarity to other bioprostheses described in the literature.
Ultrasound and electromagnetic resonance examinations proved to be ineffective methods as a tool for evaluating EMCs in the postoperative period.
“Subcutaneous/implant/aponeurosis” fixation points throughout the detached area may eliminate the need for aspiration drains in the presence of bovine pericardial bioprostheses implanted in a suprafascial position.
The use of extracellular matrices brings important conceptual developments that must be incorporated by surgeons, with the choice of bioprostheses having to be considered factors that go beyond the cost-benefit ratio of the procedures. Series with a greater number of cases and complex reconstructions will be able to define the criteria for its indication better.
REFERENCES
1. Everling EM, Bandeira DS, Gallotti FM, Bossardi P, Tonatto-Filho AJ, Grezzana-Filho TJM. Open vs laparoscopic hernia repair in the Brazilian public health system. An 11-year nationwide population-based study. Arq Gastroenterol. 2020;57(4):484-90.
2. Rohrich RJ, Lowe JB, Hackney FL, Bowman JL, Hobar PC. An algorithm for abdominal wall reconstruction. Plast Reconstr Surg. 2000;105(1):202-16.
3. Beale EW, Hoxworth RE, Livingston EH, Trussler AP. The role of biologic mesh in abdominal wall reconstruction: a systematic review of the current literature. Am J Surg. 2012;204(4):510-7.
4. Burcharth J, Pommergaard HC, Bisgaard T, Rosenberg J. Patient-related risk factors for recurrence after inguinal hernia repair: a systematic review and meta-analysis of observational studies. Surg Innov. 2015;22(3):303-17.
5. Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343(6):392-8.
6. DeBord JR. The historical development of prosthetics in hernia surgery. Surg Clin North Am. 1998;78(6):973-1006.
7. Janis JE, O’Neill AC, Ahmad J, Zhong T, Hofer SOP. Acellular dermal matrices in abdominal wall reconstruction: a systematic review of the current evidence. Plast Reconstr Surg. 2012;130(5 Suppl 2):183S-193S.
8. Wainwright D, Madden M, Luterman A, Hunt J, Monafo W, Heimbach D, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil. 1996;17(2):124-36.
9. Cicilioni O Jr, Araujo G, Mimbs N, Cox MD. Initial experience with the use of porcine acellular dermal matrix (Strattice) for abdominal wall reinforcement after transverse rectus abdominis myocutaneous flap breast reconstruction. Ann Plast Surg. 2012;68(3):265-70.
10. Armitage S, Seman EI, Keirse MJ Use of surgisis for treatment of anterior and posterior vaginal prolapse. Obstet Gynecol Int. 2012;2012:376251. DOI: 10.1155/2012/376251
11. Eichler C, Vogt N, Brunnert K, Sauerwald A, Puppe J, Warm M. A Head-to-head Comparison between SurgiMend and Epiflex in 127 Breast Reconstructions. Plast Reconstr Surg Glob Open. 2015;3(6):e439.
12. Limpert JN, Desai AR, Kumpf AL, Fallucco MA, Aridge DL. Repair of abdominal wall defects with bovine pericardium. Am J Surg. 2009;198(5):e60-5. DOI: 10.1016/j.amjsurg.2009.01.027
13. Braile Biomédica. Braile - Life-Saving Trechnologies. Nossa história [Internet]. São José do Rio Preto (SP): Braile Biomédica; 2021; [acesso em 2024 Jan 31]. Disponível em: https://braile.com.br/nossa-historia
14. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5(1):1-13.
15. Clemens MW, Selber JC, Liu J, Adelman DM, Baumann DP, Garvey PB, et al. Bovine versus porcine acellular dermal matrix for complex abdominal wall reconstruction. Plast Reconstr Surg. 2013;131(1):71-9.
16. Garvey PB, Martinez RA, Baumann DP, Liu J, Butler CE. Outcomes of abdominal wall reconstruction with acellular dermal matrix are not affected by wound contamination. J Am Coll Surg. 2014;219(5):853-64.
17. Butler CE. The role of bioprosthetics in abdominal wall reconstruction. Clin Plast Surg. 2006;33(2):199-211.
18. Law NW, Ellis H. A comparison of polypropylene mesh and expanded polytetrafluoroethylene patch for the repair of contaminated abdominal wall defects--an experimental study. Surgery. 1991;109(5):652-5.
19. Costa A, Naranjo JD, Londono R, Badylak SF. Biologic Scaffolds. Cold Spring Harb Perspect Med. 2017;7(9):a025676. DOI: 10.1101/cshperspect.a025676
20. Mioton LM, Jordan SW, Hanwright PJ, Bilimoria KY, Kim JY. The Relationship between Preoperative Wound Classification and Postoperative Infection: A Multi-Institutional Analysis of 15,289 Patients. Arch Plast Surg. 2013;40(5):522-9. DOI: 10.5999/aps.2013.40.5.522
21. Ventral Hernia Working Group; Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544-58. DOI: 10.1016/j.surg.2010.01.008
22. Awad ZT, Puri V, LeBlanc K, Stoppa R, Fitzgibbons RJ Jr, Iqbal A, et al. Mechanisms of ventral hernia recurrence after mesh repair and a new proposed classification. J Am Coll Surg. 2005;201(1):132-40.
23. Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4(12):e01067. DOI: 10.1016/j.heliyon. 2018.e01067
24. Ajdic D, Zoghbi Y, Gerth D, Panthaki ZJ, Thaller S. The Relationship of Bacterial Biofilms and Capsular Contracture in Breast Implants. Aesthet Surg J. 2016;36(3):297-309. DOI: 10.1093/asj/sjv177
25. Kao AM, Arnold MR, Augenstein VA, Heniford BT. Prevention and Treatment Strategies for Mesh Infection in Abdominal Wall Reconstruction. Plast Reconstr Surg. 2018;142(3 Suppl):149S-155S. DOI: 10.1097/PRS.0000000000004871
26. White TJ, Santos MC, Thompson JS. Factors affecting wound complications in repair of ventral hernias. Am Surg. 1998;64(3):276-80.
27. Gurusamy KS, Allen VB, Samraj K. Wound drains after incisional hernia repair. Cochrane Database Syst Rev. 2012;15(2):CD005570. DOI: 10.1002/14651858.CD005570.pub3
28. Baroudi R, Ferreira CA. Seroma: how to avoid it and how to treat it. Aesthet Surg J. 1998;18(6):439-41. DOI: 10.1016/s1090-820x(98)70073-1
29. Kingsnorth A. The management of incisional hernia. Ann R Coll Surg Engl. 2006;88(3):252-60.
30. Korenkov M, Paul A, Sauerland S, Neugebauer E, Arndt M, Chevrel JP, et al. Classification and surgical treatment of incisional hernia. Results of an experts’ meeting. Langenbecks Arch Surg. 2001;386(1):65-73.
31. Jin J, Rosen MJ, Blatnik J, McGee MF, Williams CP, Marks J, et al. Use of acellular dermal matrix for complicated ventral hernia repair: does technique affect outcomes? J Am Coll Surg. 2007;205(5):654-60. DOI: 10.1016/j.jamcollsurg.2007.06.012
32. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516.
33. Smart NJ, Bloor S. Durability of biologic implants for use in hernia repair: a review. Surg Innov. 2012;19(3):221-9.
34. de Castro Brás LE, Shurey S, Sibbons PD. Evaluation of crosslinked and non-crosslinked biologic prostheses for abdominal hernia repair. Hernia. 2012;16(1):77-89.
35. Mestak O, Spurkova Z, Benkova K, Vesely P, Hromadkova V, Miletin J, et al. Comparison of Cross-linked and Non-Cross-linked Acellular Porcine Dermal Scaffolds for Long-term Full-Thickness Hernia Repair in a Small Animal Model. Eplasty. 2014;14:e22.
36. Gaertner WB, Bonsack ME, Delaney JP. Experimental evaluation of four biologic prostheses for ventral hernia repair. J Gastrointest Surg. 2007;11(10):1275-85.
37. Smart NJ, Daniels IR, Marquez S. Supplemental cross-linking in tissue-based surgical implants for abdominal wall repair. Int J Surg. 2012;10(9):436-42. DOI: 10.1016/j.ijsu.2012.07.010
38. Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88(12):2673-86.
39. Catena F, Ansaloni L, Gazzotti F, Gagliardi S, Di Saverio S, D’Alessandro L, et al. Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia. 2007;11(1):57-60.
40. Sartelli M, Coccolini F, van Ramshorst GH, Campanelli G, Mandalà V, Ansaloni L, et al. WSES guidelines for emergency repair of complicated abdominal wall hernias. World J Emerg Surg. 2013;8(1):50. DOI: 10.1186/1749-7922-8-50
41. Courtman DW, Pereira CA, Kashef V, McComb D, Lee JM, Wilson GJ. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J Biomed Mater Res. 1994;28(6):655-66.
42. Liang HC, Chang Y, Hsu CK, Lee MH, Sung HW. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials. 2004;25(17):3541-52. DOI: 10.1016/j.biomaterials.2003.09.109
43. Hoganson DM, Owens GE, O’Doherty EM, Bowley CM, Goldman SM, Harilal DO, et al. Preserved extracellular matrix components and retained biological activity in decellularized porcine mesothelium. Biomaterials. 2010;31(27):6934-40.
44. Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ, et al. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016;2(6):e1600502.
45. Crapo PM, Tottey S, Slivka PF, Badylak SF. Effects of biologic scaffolds on human stem cells and implications for CNS tissue engineering. Tissue Eng Part A. 2014;20(1-2):313-23.
46. Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014;163(4):268-85.
47. Langer C, Schaper A, Liersch T, Kulle B, Flosman M, Füzesi L, et al. Prognosis factors in incisional hernia surgery: 25 years of experience. Hernia. 2005;9(1):16-21. DOI 10.1007/s10029-004-0265-y
1. Hospital do Coração de São José do Rio Preto,
São José do Rio Preto, SP, Brazil
2. Sociedade Portuguesa de Beneficência de São
José do Rio Preto, São José do Rio Preto, SP, Brazil
3. Hospital Estadual João Paulo II, São José do
Rio Preto, SP, Brazil
Corresponding author: Luiz Fernando Frascino Av. Juscelino Kubitscheck de Oliveira, 3700, São José do Rio Preto, SP, Brazil, Zip Code: 15093-225, E-mail: drfrascino@gmail.com
Article received: March 14, 2023.
Article accepted: October 23, 2023.
Conflicts of interest: none.





 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter