

Original Article - Year 2024 - Volume 39 -
Cryopreservation of adipose tissue-derived mesenchymal stem cells: An alternative to serial fat grafting in breast reconstruction
Criopreservação de células-tronco mesenquimais do tecido adiposo: Uma alternativa para lipoenxertia seriada na reconstrução mamária
ABSTRACT
Introduction: Fat grafting is an autologous graft of cells from subcutaneous tissue, which can be used as a complementary technique in breast reconstruction. Given this, the cryopreservation of adipose tissue-derived mesenchymal stem cells (ADMSCs) could be a way to collect them in one surgical procedure and after performing fractional fat grafting. Dimethyl sulfoxide (DMSO) is a cryopreservative used in cell research, but it is potentially toxic, which would make it impossible to use cryopreserved ADMSCs in clinical practice. New cellular cryopreservatives, without toxicity, have been described in the experimental scientific literature, such as the substances L-proline and trehalose. Therefore, this work aimed to evaluate the viability of ADMSCs cryopreserved with the combination of L-proline and trehalose over up to 90 days.
Method: Experimental study in which lipoaspirate samples were obtained from 9 patients. The cellular fraction was processed and frozen with L-proline (1.5M) + trehalose (0.2M) or with DMSO + fetal bovine serum (FBS) as control. After 30 and 90 days, the samples were thawed, and cell viability was assessed using the MTT technique.
Results: The analysis of ADMSCs, after 1 and 3 months of freezing, indicated that samples treated with L-proline + trehalose showed similar viability to those preserved with DMSO and SFB (p=0.444).
Conclusion: The association of L-proline and trehalose kept ADMSC viable for 30 and 90 days of freezing, and could be an alternative as a cellular cryopreservative without toxicity and enabling the use of serial fat grafting.
Keywords: Trehalose; Lipectomy; Breast neoplasms; Mesenchymal stem cells; Adipocytes; Proline
RESUMO
Introdução: A lipoenxertia é um enxerto autólogo de células do tecido celular subcutâneo, que pode ser utilizada como técnica complementar na reconstrução mamária. Diante disso, a criopreservação de células-tronco mesenquimais provenientes de tecido adiposo (CTDAs) poderia ser uma maneira de realizar a coleta em um tempo cirúrgico e após realizar a lipoenxertia de forma fracionada. O dimetilsulfóxido (DMSO) é um criopreservante utilizado em pesquisas com células, porém é potencialmente tóxico, o que impossibilitaria a utilização de CTDAs criopreservadas na prática clínica. Novos criopreservantes celulares, sem toxicidade, vêm sendo descritos na literatura científica experimental, como as substâncias L-prolina e trealose. Com isso, esse trabalho teve como objetivo avaliar a viabilidade de CTDAs criopreservadas com a combinação de L-prolina e trealose, em um período de até 90 dias.
Método: Estudo experimental, no qual foram obtidas amostras de lipoaspirado provenientes de 9 pacientes. A fração celular foi processada e congelada com L-prolina (1,5M) + trealose (0,2M), ou com DMSO + soro fetal bovino (SFB), como controle. Após 30 e 90 dias, as amostras foram descongeladas e a viabilidade celular foi avaliada pela técnica de MTT.
Resultados: A análise das CTDAs, após 1 e 3 meses de congelamento, indicou que as amostras tratadas com L-prolina + trealose apresentaram viabilidade semelhante àquelas preservadas com DMSO e SFB (p=0,444).
Conclusão: A associação de L-prolina e trealose manteve CTDA viáveis por 30 e 90 dias de congelamento, podendo ser uma alternativa como criopreservante celular sem toxicidade e viabilizando o uso de lipoenxertia seriada.
Palavras-chave: Trealose; Lipectomia; Neoplasias da mama; Células-tronco mesenquimais; Adipócitos. Prolina
INTRODUCTION
Surgical breast reconstruction, after locoregional cancer treatment, is related to patients’ quality of life1. However, in a country like Brazil, access to this type of procedure for the vast majority of women affected by breast cancer is very limited, considering that more than one intervention is often necessary to achieve the expected aesthetic result.
Thinking about the accessibility of breast repair surgery in the Brazilian public health system, Law No. 9,797, of May 6, 1999, defined the obligation of this type of procedure by the network of units that are part of the Unified Health System (SUS) in cases of mutilation resulting from cancer treatment2. This legislation was modified in 2013 (by Law No. 12,802), establishing that aesthetic repair should be performed at the same surgical time as cancer treatment, and when this was impossible, breast reconstruction would be guaranteed in a second stage3; and by ordinance GM/MS nº 127, of February 2023, which decided to install an exceptional strategy to expand access to breast reconstruction4.
There are numerous surgical treatment options for breast cancer, and the technique used depends on several factors, which include the biological characteristics of the breast neoplasm, the volume of the breast, the dimensions of the primary lesion, the response to neoadjuvant systemic treatment and the stage of the disease at the time of diagnosis, among others5. Also, with greater access to genetic tests to check the presence of pathogenic variants of genes related to a greater predisposition to breast cancer, such as BRCA1 and BRCA2, the indication for prophylactic adenomastectomies has increased6.
Fat grafting (or lipofilling) is an autologous graft of adipose tissue cells, widely used as an adjuvant method in breast reconstruction after oncological surgery and/or radiotherapy to correct volume and irregularities7. Recently, it was demonstrated that the use of fat grafting is safe, including in conservative surgeries for the treatment of breast cancer, and can be used at the same surgical time as the excision of breast neoplasia8,9. Associated with the indications for this technique in the oncology area, it is also used in numerous aesthetic procedures in the face and breast region, in remodeling contours in different anatomical sites, in addition to being used to alleviate scar contractures in large burns10,11.
This type of graft is a complementary method that allows obtaining large quantities of mesenchymal stem cells derived from subcutaneous adipose tissue (ADMSCs), which are the cells responsible for colonization and tissue formation in the recipient area12,13. Currently, ADMSCs are the most used as a cell renewal strategy, as they have a similar capacity to stem cells originating from the bone marrow, in addition to being obtained through a less traumatic and invasive technique. However, the procedure has limitations, as part of the volume of fat applied is reabsorbed, compromising the result14.
In breast reconstruction, fat grafting is performed immediately after liposuction of small volumes of cellular fraction, with no preservation of material for subsequent grafts, with the surplus discarded. In situations where there is a need for larger volumes of fat grafting, several sequential fat grafting procedures are performed until the expected aesthetic result is achieved15. This increases operating costs, in addition to generating greater surgical risks for the patient.
The possibility of using cell cryopreservation techniques could help in this process, as the ADMSCs would be preserved at low temperatures, for an indefinite period and be thawed in fractions depending on the surgical planning16. Cell cryopreservation seeks to ensure adequate cell viability rates while maintaining their biological potential. To achieve this, it is necessary to use cryopreservatives during freezing techniques, with dimethyl sulfoxide (DMSO) being the substance most used in conventional protocols in experimental studies, as it provides good cell viability after thawing17. However, the use of cryopreservation of ADMSCs with DMSO in clinical practice is limited, as this solvent is toxic to human cells18.
Considering the importance of ADMSCs in post-mastectomy breast reconstruction, among other indications in aesthetic surgery, the search for non-toxic and effective cryopreservatives would allow the conservation of ADMSCs and, consequently, the planning of serial fat grafts.
OBJECTIVE
Verify the cell viability of ADMSCs after cryopreservation of lipoaspirates with the combination of L-proline and trehalose, substances that have no documented cellular toxicity, in addition to having a low operational cost.
METHOD
Experimental cross-sectional descriptive study in which lipoaspirated tissue samples were collected from female patients over 18 years of age who signed the Free and Informed Consent Form (FICF) and chose to donate part of the lipoaspirate that would be discarded after the aesthetic liposuction procedure; carried out by 4 plastic surgeons in hospitals in the city of Chapecó-SC, between June 2021 and March 2022.
Seeking to compare the effectiveness of cryopreservatives against different freezing times, 90mL of lipoaspirated material was collected from each patient, which was then allocated into 6 15mL Falcon® tubes. The material was centrifuged at 3000 rpm for 3 minutes, washed with 0.9% saline solution, and centrifuged again with the same parameters. Successively, collagenase IA (C2674 - Sigma®) was added at a concentration of 0.075% in a proportion of 1:100, followed by incubation in a water bath at 37ºC for 30 minutes, with homogenization every 10 minutes.
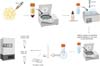
Collagenase inactivation was carried out with the addition of DMEM (Dulbecco’s Modified Eagle’s Medium) + 10% fetal bovine serum (FBS) in a 1:1 ratio, and the content was then centrifuged at 1600rpm for 10 minutes. The supernatant was discarded, and the cell pellet was transferred to a new tube and resuspended in 2mL of phosphate buffered saline (PBS- Phosphate Buffered Saline) for subsequent cell counting in a Neubauer chamber. The cell set was divided into 6 tubes, and DMSO was added to 3 of them for a final concentration of 10% + 10% FBS. In the remaining tubes, 1.5M L-proline and 0.2M trehalose dissolved in PBS buffer were added. The samples were then frozen at -80ºC (Figure 1).
The sample thawing process in a water bath at 37ºC took place after 30 and 90 days. In samples containing DMSO, removal was performed by dilution and washing with PBS buffer. L-proline and trehalose did not require removal. The samples were cultured in triplicate in sterile 12-well plates at a concentration of 106 cells per well. 2mL of DMEM containing 10% FBS and 1% penicillin + 1% streptomycin were added to each well. The samples were incubated in a CO2-free oven at 37ºC for 24 hours.
Cell viability was performed using the 3-(4,5 - dimethylthiazol-2yl)-2,5 diphenyltetrazolium bromide (MTT) assay. The content contained in the wells was transferred to 1.5mL microtubes, which were centrifuged at 3000rpm for 15 minutes. The supernatant portion was discarded, and 300uL of PBS + 20uL of MTT were added to the cell pellet and incubated again at 37ºC for 1 hour. Subsequently, the samples were centrifuged with the same parameters as in the previous step, then 75uL of each sample was discarded, and the same volume of DMSO was added. The samples were homogenized and transferred to a 96-well plate for absorbance evaluation by spectrophotometry at 560ηm (Figure 2).
The cell viability data generated were tabulated in a Microsoft® Office Excel spreadsheet, and a two-way ANOVA test was then performed using GraphPad Prism 6.0, considering the results significant when p<0.05.
The initial research project was approved by the Unochapecó Research Ethics Committee, under CAAE 23243519.7.0000.0116, and opinion number 4,822,999, and by the institutions involved.
RESULTS
Samples were processed and frozen from 9 female patients, self-declared as white, and who underwent a third generation ultrasonic assisted liposuction procedure (Vaser). The lipoaspirates were cryopreserved and analyzed in two moments, after 30 and 90 days of freezing. The average age of the liposuction donor patients was 35.7 years, while the average body mass index (BMI) was 25kg/m2.
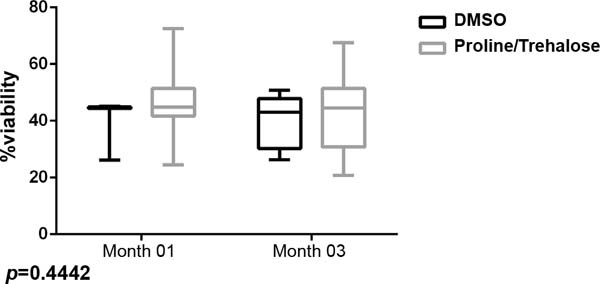
The main collection site was in the abdominal region and the cannula number most used was 3.7mm. The assessment of cell viability after one and three months of freezing indicated that samples cryopreserved with L-proline + trehalose showed cell viability similar to those containing DMSO + SBF (p=0.4442) (Figure 3). Furthermore, in relation to freezing time, samples that were frozen for 90 days maintained viable ADMSCs when compared to samples thawed in the first month (p=0.5301), noting that, in addition to the type of cryopreservative, the freezing time also did not seem to interfere with cell viability.
DISCUSSION
Several factors can influence the viability of ADMSCs, including lipoaspirate extraction and injection methodologies, centrifugation speed, use of anesthetic and/or saline solutions, the volume of aspirated content, location and biological characteristics of the adipose tissue in the donor area, among others12,19. Furthermore, there is no consensus on the best way to process fat for later use as a graft11,17.
Clinical and phenotypic characteristics of patients can influence the quality of the adipose material aspirated. As in BMI, where, in patients with values greater than 25kg/m2, there is an indirect relationship with the capacity for cell proliferation and differentiation; that is, the patient’s excess weight can contribute to unsatisfactory results when using ADMSCs in reconstructive surgeries20.
The patient’s age is also a relevant factor due to the changes caused by aging in ADMSCs. This is due to several cellular events, such as the shortening of telomeres and weakening of the antioxidant protection system associated with oxidative stress and accumulated damage to the DNA repair system21,22.
In the present study, these factors probably did not interfere in the assessment of cell viability, as the average values found for these two variables in the group of patients studied were within the range of results described in the reviewed literature19-21.
Another aspect verified in the literature that could interfere with cell viability would be the diameter of the cannula used both in collecting the lipoaspirate in the donor area and in injecting the fraction containing the ADMSCs in the recipient area23. In a review article, it was shown that most authors use cannulas ranging from 2mm to 6mm24, but cell viability is greater when liposuction is performed with cannulas with diameters greater than 3mm25. These results can be explained by obtaining aspirates with a large number of cells from the stromal vascular fraction, compared to the use of cannulas with smaller diameters25,26.
Most authors use cannulas with a diameter smaller than 2mm to inject the intermediate fraction of the lipoaspirate, as this reduces the chance of material extravasation, in addition to minimizing the possibility of injecting the content intravascularly27. In the present study, samples obtained with 3mm, 3.7mm, 4mm, and 5mm cannulas were used, with the 3.7mm being the most prevalent. Fat grafting was not performed in any of the procedures.
The way the lipoaspirate components are separated can be via decantation or centrifugation. The centrifugation technique allows you to concentrate fat and increase the number of cells per milliliter while separating the liquefied fat and blood cell components28. Studies indicate that the ADMSCs present in the sediment, obtained after washing the centrifuged material, have greater viability since they are not contaminated with blood cell residues, which is common in samples treated only by decantation, in addition to there being greater disruption of the adipocyte walls and a greater number of ADMSCs12,29,30.
In the publication in which we compared different centrifugation speeds, we documented the methodology described by Coleman (3000rpm for three minutes) and found greater maintenance of the biological characteristics of ADMSCs necessary for cell colonization in graft recipient areas12,29. This is because centrifuging the samples makes the fat cells more concentrated, in addition to separating them from the blood cells30.
Likewise, in the present study, the samples were centrifuged to separate the tissue fractions into three well-defined portions: a lower layer of blood cells, an intermediate layer containing stromal cells and adipose tissue, and a superficial layer of liquefied fat. As a result, there was no interference from blood cell debris and liquefied fat in the cultivation and cryopreservation of ADMSCs.
Cell viability after a period of cryopreservation may also vary depending on the number of cells stored in each microtube31. Goh et al.16 used four cell concentrations and noted that the concentration of 5x105 cells/mL presented a viability rate of 81.10%, while at a concentration of 1x106 cells/mL, the viability was 77.9%. Few studies described in the literature have documented the viability of ADMSCs as significant as the one published by De Rosa et al.32, in which a rate of 92.5% of viable cells was described; however, the cell concentration was 6x103 cells/mL.
For this research, the concentration of cells used in each microtube was 5x105 cells/mL, and the viability rates in cells frozen with both L-proline and trehalose and the DMSO control were close to 60%. Comparing these results, it is possible to infer that a smaller number of cells stored in each microtube is related to a higher viability rate, probably due to the more adequate space for the physiological maintenance of ADMSCs.
Considering the temperature used to freeze the samples, we kept the cells frozen at -80ºC, using the rapid cooling technique, which was also used by Ray et al.33. However, currently, the most frequently described temperature for cell storage would be -196ºC (via liquid nitrogen), as the use of a -80ºC freezer would be related to the greater formation of ice crystals, which could cause damage to the cryopreserved material. However, the use of liquid nitrogen is a technique that requires high maintenance costs31,34.
In relation to cryopreservatives, DMSO is the substance most used in various protocols for cell preservation, as it is related to maintaining the viability of CTAS11. However, it is a toxic substance causing negative changes in cellular processes such as metabolism, citric acid cycle pathways, respiratory electron transport, glucose, lipid, and lipoprotein metabolism. It is also related to changes in mitochondrial pathways, the production of reactive oxygen species, and the generation of cellular ATP35.
In the present research, we chose to use two low-cost natural cryopreservatives, which are alternatives to DMSO. Trehalose is a non-reducing disaccharide made up of two glucose units, while L-proline is a natural amino acid formed through biosynthesis from L-glutamate. Both do not cause toxicity in the cell and are a source of studies in the search for a suitable cryopreservative36.
In a study carried out with red blood cells cryopreserved with different concentrations of L-proline and trehalose, maintenance of cellular structure was observed, in addition to not having altered the activity of the Na +/K +-ATPase pumps, whose function is to maintain the concentrations of intracellular ions, very relevant for signal transduction and cellular metabolism, in addition to DNA methylation not being altered22,37. Furthermore, because L-proline and trehalose are not toxic to cells, there is no need to remove cryoprotectants, reducing cell loss, as is done when DMSO23 is used.
According to studies by Dovgan et al.38, which used a cryopreservation protocol with only trehalose (0.25M), there was comparable cell viability of the non-toxic agent in relation to DMSO; it was also observed that trehalose concentrations have a positive correlation in relation to cell viability. In another study, it was demonstrated that ADMSCs from lipoaspirate, when cryopreserved for 6 months with trehalose at a concentration of 0.35M, are able to maintain biological activity, almost at the same rate as fresh tissue39. In the present study, the association of L-proline and trehalose showed cell vitality levels comparable to the use of DMSO in the cryopreservation of ADMSCs.
Cell cryopreservation time has also been studied31,34,40. De Rosa et al.32 kept the cells frozen for up to 12 months, using trehalose, DMSO, and SBF, and found significant cell viability data, with more than 80% of cells recovered. Also, it has already been documented that, when leaving the cells cryopreserved for 6 months, the cell proliferation and differentiation potential was similar to the group of non-cryopreserved cells39,41. In the present study, it was possible to demonstrate that ADMSC samples frozen for 90 days showed cell viability similar to those frozen for 30 days. In other words, cryopreservation for long periods can be a feasible methodology, for the fractional use of ADMSCs in multiple surgical times with the use of sufficient volumes for surgical corrections, whether aesthetic or repair.
CONCLUSION
Cryopreservation of ADMSCs with the combined use of L-proline and trehalose resulted in cell viability equivalent to the use of DMSO. Furthermore, cells frozen for 90 days maintained viability rates similar to those stored for a shorter period. Considering the data found and those reviewed in the literature, the use of the combination of L-proline and trehalose may be an option for DMSO, avoiding cellular toxicity. This methodology can be an alternative to avoid multiple liposuction collections in surgical procedures that require several fat grafting sessions, especially in breast reconstruction after surgical treatment of breast cancer.
ACKNOWLEDGMENTS
To all fellow researchers in the research group “Molecular Biology and Biotechnology in Health” at the Universidade Comunitária da Região de Chapecó (Unochapecó) and the research group “Biological and Clinical Studies in Human Pathologies” at the Universidade Federal da Fronteira Sul (UFFS) Chapecó-SC campus. To plastic surgeons Dr. Gustavo Colonheze, Dr. Jorge Diego Valentini, Dr. Rafael de Almeida Tirapelle, and Dr. Tainara Cassol, who kindly agreed to participate in the initial contact of the patients and welcome the team of researchers.
REFERENCES
1. Fanakidou I, Zyga S, Alikari V, Tsironi M, Stathoulis J, Theofilou P. Mental health, loneliness, and illness perception outcomes in quality of life among young breast cancer patients after mastectomy: the role of breast reconstruction. Qual Life Res. 2018;27(2):539-43.
2. Brasil. Presidência da República. Lei Nº 9.797, de 6 de maio de 1999. Dispõe sobre a obrigatoriedade da cirurgia plástica reparadora da mama pela rede de unidades integrantes do Sistema Único de Saúde - SUS nos casos de mutilação decorrentes de tratamento de câncer. Brasília: Presidência da República; 1999. [Internet]. Disponível em: https://legislacao.presidencia.gov.br/atos/?tipo=LEI&numero=9797&ano=1999&ato=b04cXRE9keNpWT174
3. Brasil. Presidência da República. Lei Nº 12.802, de 24 de abril de 2013. Altera a Lei nº 9.797, de 6 de maio de 1999, que “dispõe sobre a obrigatoriedade da cirurgia plástica reparadora da mama pela rede de unidades integrantes do sistema único de saúde - SUS nos casos de mutilação decorrentes de tratamento de câncer”, para dispor sobre o momento da reconstrução mamária. [Internet]. Brasília: Presidência da República; 2013. Disponível em: https://legislacao.presidencia.gov.br/atos/?tipo=LEI&numero=12802&ano=2013&ato=97cATRU50MVpWTabf
4. Brasil. Ministério da Saúde. Portaria GM/MS nº 127, de 13 de fevereiro de 2023. Institui estratégia excepcional de ampliação do acesso à reconstrução mamária em caso de mulheres com diagnóstico de câncer de mama, no âmbito do Sistema Único de Saúde -SUS. [Internet]. Brasília: Ministério da Saúde; 2023. Disponível em: https://www.gov.br/saude/pt-br/composicao/saes/legislacao/portaria-gm-ms-no-127-de-13-de-fevereiro-de-2023/view
5. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750-69.
6. Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-Associated Hereditary Breast and Ovarian Cancer: Synonym: BRCA1- and BRCA2-Associated HBOC [Internet]. 2022 [acesso 2022 nov 11]. Disponível em: https://www.ncbi.nlm.nih.gov/books/NBK1247/
7. Tan SS, Loh W. The utility of adipose-derived stem cells and stromal vascular fraction for oncologic soft tissue reconstruction: Is it safe? A matter for debate. Surgeon. 2017;15(4):186-9.
8. Stumpf CC, Zucatto ÂE, Cavalheiro JAC, de Melo MP, Cericato R, Damin APS, et al. Oncologic safety of immediate autologous fat grafting for reconstruction in breast-conserving surgery. Breast Cancer Res Treat. 2020;180(2):301-9.
9. Krastev TK, Schop SJ, Hommes J, Piatkowski AA, Heuts EM, van der Hulst RRWJ. Meta-analysis of the oncological safety of autologous fat transfer after breast cancer. Br J Surg. 2018;105(9):1082-97.
10. He X, Zhang J, Luo L, Shi J, Hu D. New Progress of Adipose-derived Stem Cells in the Therapy of Hypertrophic Scars. Curr Stem Cell Res Ther. 2020;15(1):77-85.
11. Zuk P. Adipose-Derived Stem Cells in Tissue Regeneration: A Review. Int Sch Res Notices. 2013;2013:1-35.
12. Moreno M, Schmidt JC, Gazzoni CD, Dal-Magro L, Bonadiman BDSR, Kosvoski GC, et al. Viability of mesenchymal stem cells of adipose tissue from human liposuction. Rev Bras Cir Plást. 2021;36(1):9-14.
13. da Silva A, do Prado J, Pignataro J, Moreno M. Lipoenxertia. In: Bagnoli F, Postiglione F, Palermo F, Pedrini JL, Freitas Júnior R, Marques V. Mastologia: do diagnóstico ao tratamento. 2ª ed. Goiânia: Conexão Soluções Corporativas; 2022.
14. Arshad Z, Karmen L, Choudhary R, Smith JA, Branford OA, Brindley DA, et al. Cell assisted lipotransfer in breast augmentation and reconstruction: A systematic review of safety, efficacy, use of patient reported outcomes and study quality. JPRAS Open. 2016;10:5-20.
15. Piffer A, Aubry G, Cannistra C, Popescu N, Nikpayam M, Koskas M, et al. Breast Reconstruction by Exclusive Lipofilling after Total Mastectomy for Breast Cancer: Description of the Technique and Evaluation of Quality of Life. J Pers Med. 2022;12(2):153.
16. Goh BC, Thirumala S, Kilroy G, Devireddy RV, Gimble JM. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1(4):322-4.
17. Lee JE, Kim I, Kim M. Adipogenic differentiation of human adipose tissue-derived stem cells obtained from cryopreserved adipose aspirates. Dermatol Surg. 2010;36(7):1078-83.
18. Wang C, Xiao R, Cao YL, Yin HY. Evaluation of human platelet lysate and dimethyl sulfoxide as cryoprotectants for the cryopreservation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2017;491(1):198-203.
19. Kim IH, Yang JD, Lee DG, Chung HY, Cho BC. Evaluation of centrifugation technique and effect of epinephrine on fat cell viability in autologous fat injection. Aesthet Surg J. 2009;29(1):35-9.
20. Frazier TP, Gimble JM, Devay JW, Tucker HA, Chiu ES, Rowan BG. Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue-derived stem cells. BMC Cell Biol. 2013;14:34.
21. Efimenko AY, Kochegura TN, Akopyan ZA, Parfyonova YV. Autologous Stem Cell Therapy: How Aging and Chronic Diseases Affect Stem and Progenitor Cells. Biores Open Access. 2015;4(1):26-38.
22. Zhang TY, Tan PC, Xie Y, Zhang XJ, Zhang PQ, Gao YM, et al. The combination of trehalose and glycerol: an effective and non-toxic recipe for cryopreservation of human adipose-derived stem cells. Stem Cell Res Ther. 2020;11(1):460.
23. Toledo LS, Mauad R. Fat injection: a 20-year revision. Clin Plast Surg. 2006;33(1):47-53.
24. Piccotti F, Rybinska I, Scoccia E, Morasso C, Ricciardi A, Signati L, et al. Lipofilling in Breast Oncological Surgery: A Safe Opportunity or Risk for Cancer Recurrence? Int J Mol Sci. 2021;22(7):3737.
25. Becker H, Vazquez OA, Rosen T. Cannula Size Effect on Stromal Vascular Fraction Content of Fat Grafts. Plast Reconstr Surg Glob Open. 2021;9(3):e3471.
26. Tong Y, Liu P, Wang Y, Geng C, Han X, Ma J, et al. The Effect of Liposuction Cannula Diameter on Fat Retention-Based on a Rheological Simulation. Plast Reconstr Surg Glob Open. 2018;6(11):e2021.
27. Biazus JV, Falcão CC, Parizotto AC, Stumpf CC, Cavalheiro JA, Schuh F, et al. Immediate Reconstruction with Autologous fat Transfer Following Breast-Conserving Surgery. Breast J. 2015;21(3):268-75.
28. Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63(8):1375-81.
29. Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19(5):421-5.
30. Ladeira PRS, Isaac C, Nakamura YM, Tutihashi RMC, Paggiaro AO, Ferreira MC. Cultivo de células-tronco derivadas de tecido adiposo: uma análise crítica. Rev Med (São Paulo). 2012;91(4):246-52.
31. Irioda AC. Avaliação da integridade das células-tronco mesenquimais derivadas do tecido adiposo humano após o bioprocesso de criopreservação [Dissertação de mestrado]. Curitiba: Universidade Federal do Paraná; 2010. Disponível em: http://hdl.handle.net/1884/26521
32. De Rosa A, De Francesco F, Tirino V, Ferraro GA, Desiderio V, Paino F, et al. A new method for cryopreserving adipose-derived stem cells: an attractive and suitable large-scale and long-term cell banking technology. Tissue Eng Part C Methods. 2009;15(4):659-67.
33. Ray SS, Pramanik K, Sarangi SK, Jain N. Serum-free non-toxic freezing solution for cryopreservation of human adipose tissue-derived mesenchymal stem cells. Biotechnol Lett. 2016;38(8):1397-404.
34. Ginani F, Soares DM, Barboza CAG. Influência de um protocolo de criopreservação no rendimento in vitro de células-tronco derivadas do tecido adiposo. Rev Bras Cir Plást. 2012;27(3):359-63.
35. Verheijen M, Lienhard M, Schrooders Y, Clayton O, Nudischer R, Boerno S, et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci Rep. 2019;9(1):4641.
36. Kennelly P, Rodwell V. Aminoácidos e Peptídeos. In: Rodwell VW, Bender D, Botham KM, Kennelly PJ, Weil PA. Bioquímica Ilustrada de Harper. 31ª ed. Porto Alegre: AMGH Editora; 2021.
37. Dou M, Lu C, Sun Z, Rao W. Natural cryoprotectants combinations of l-proline and trehalose for red blood cells cryopreservation. Cryobiology. 2019;91:23-9.
38. Dovgan B, Miklavčič D, Knežević M, Zupan J, Barlič A. Intracellular delivery of trehalose renders mesenchymal stromal cells viable and immunomodulatory competent after cryopreservation. Cytotechnology. 2021;73(3):391-411.
39. Dalmagro JP. Isolamento e caracterização das células-tronco mesenquimais provenientes do tecido adiposo e avaliação da sua viabilidade tecidual após criopreservação [Monografia]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2019.
40. Lenoch CY. Estratégias para criopreservação de células tronco mesenquimais de tecido adiposo bovino [Dissertação de mestrado]. Lages: Universidade do Estado de Santa Catarina; 2015.
41. Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, Inoue K, et al. Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg. 2008;121(2):401-10.
1. Universidade Federal da Fronteira Sul, Chapecó,
SC, Brazil
2. Universidade Comunitária da Região de Chapecó,
Chapecó, SC, Brazil
Corresponding author: Marcelo Moreno Rodovia SC 484, Km 02, Bloco dos professores, Chapecó, SC, Brazil, Zip Code: 89815-899, E-mail: marcelo.moreno@uffs.edu.br
Article received: May 15, 2023.
Article accepted: August 20, 2023.
Conflicts of interest: none.






 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter