INTRODUCTION
A wound is defined as the loss of skin coverage, representing a break in the
continuity of normal tissue structures and functions, and may affect not only
the skin but also the subcutaneous tissue, muscles, and bones.1 Many of these wounds represent a
challenge for medical and nursing teams, being difficult to resolve using
conventional treatments and simple dressings. These cases are classified as
”complex wounds” and must be treated in a specialized hospital center and by
a
multidisciplinary team.2
Complex wounds cause high morbidity and mortality and have been identified as
a
serious public health problem in many centers.3
There are numerous techniques described for the treatment of complex wounds.
However, several of them are not fully reproducible in many centers due to the
complexity of their execution and/or cost.4
OBJECTIVE
This study aimed to describe a third intention wound healing technique,
reproducible and inexpensive, applicable to complex wounds, using a polyvinyl
chloride (PVC) prosthesis temporarily placed in the area of injury to promote
the protection and stimulate its ”granulation,” followed by autologous
partial-thickness skin grafting.
METHOD
After approval by the Research Ethics Committee (CEP) (CAAE
53971021.8.0000.5294), a prospective clinical study was carried out in the
Trauma sector of a tertiary regional hospital (Hospital Tarcísio de Vasconcelos
Maia - HRTVM -, Mossoró, RN, Brazil), between February and September 2022.
Consecutively, 20 patients with complex wounds resulting from external causes,
which mainly affect the skin/tegument, subcutaneous tissue, aponeurosis/fascia,
and muscle, were selected. These patients were divided into 2 groups: A -
patients who underwent the PVC prosthesis coverage technique, followed by
grafting, and B - patients submitted to the care of the dressing team, with
daily changes until wound granulation, standard in our institution.
The following patients were excluded: with mucosal involvement; lesion in the
genital region; injury to the face and skull; injury with tendon exposure;
injury with bone exposure; injury with exposure of the peritoneal and/or pelvic
cavity; injury with exposure of the pleural and/or mediastinal cavity; presence
of infections; previous surgeries in the injured region; systemic diseases that
significantly compromise immunity, such as decompensated diabetes, acquired
immunodeficiency syndrome, psoriasis, lupus erythematosus, rheumatoid arthritis,
tumors, among others.
Patients were evaluated regarding length of stay; hospitalization costs;
concerning local pain according to the visual analog scale for pain5, graduated from 0 to 10; the
presence of complications; the time until medical discharge; and patient
satisfaction (measured by a Likert-type scale6: In general, what is your level of satisfaction or
dissatisfaction with the evolution of your injury? 5 - Very satisfied; 4 - More
or less satisfied; 3 - Neither satisfied nor dissatisfied; 2 - More or less
dissatisfied; and 1 - Very dissatisfied).
Description of technique A
First part: coverage with PVC prosthesis
After anesthesia, asepsis, and antisepsis, the wound is carefully
debrided to leave it with a minimum of devitalized tissue and as clean
as possible. The extent of the wound is determined immediately after
proper debridement.
Then, the wound is covered with polyvinyl chloride (PVC) prosthesis
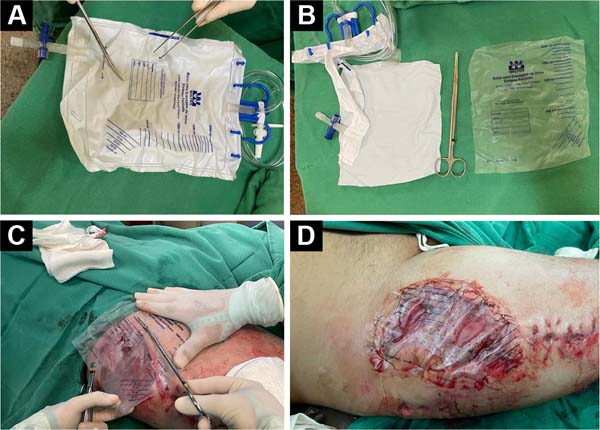
obtained from a sterile closed-system urine collection bag (Figure 1A). This collection bag is
made of flexible, double-sided PVC, with the front side usually
transparent and the back white. This material is easily accessible in
surgical centers (Figure 1B).
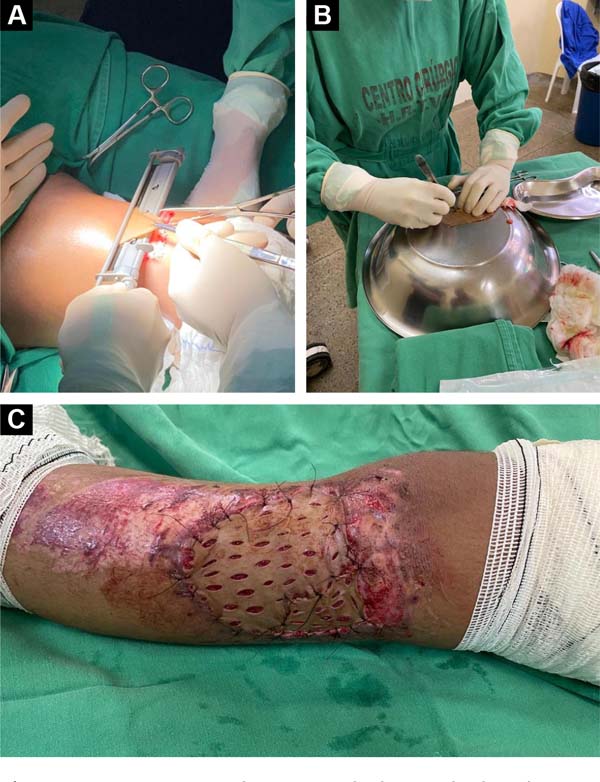
Figure 1 - A e B: Sterile closed system urine
collection bag (PVC prosthesis); C: The PVC
prosthesis is cut in a similar shape to the debrided area of
the wound; D: The PVC prosthesis is sutured to
the healthy edges of the wound.
Figure 1 - A e B: Sterile closed system urine
collection bag (PVC prosthesis); C: The PVC
prosthesis is cut in a similar shape to the debrided area of
the wound; D: The PVC prosthesis is sutured to
the healthy edges of the wound.
The bag (PVC prosthesis) is then cut in a similar shape and 0.5cm larger
than the debrided area of the wound. Then, the prosthesis is sutured to
the healthy edges of the lesion with simple stitches (Nylon 3-0 thread)
to perfectly fit the prosthesis without exerting pressure on the wound,
that is, functioning, more or less, as a semi-occlusive
dressing.7.
The dressing comprises sterile gauze and a crepe bandage covering the
prosthesis. Liquid exudate forms in this initial phase, slightly wetting
the dressing. After the first week, the exudate decreases significantly,
forming fibrin tissue, which will be gradually replaced by granulation
tissue, thus filling the area lost in the original wound format (healing
by second intention). Dressings are changed daily (local cleaning of
adjacent skin and PVC prosthesis with chlorhexidine, later covered with
sterile gauze and crepe dressing). The patient is discharged after three
days, and the dressings are changed in outpatient consultations.
Second part: partial skin graft
The prosthesis is removed after six to eight weeks, with granulation
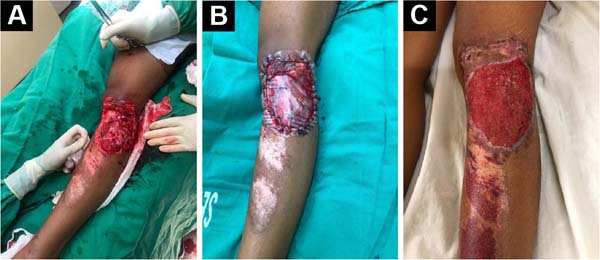
tissue filling the initial wound (Figure 2). The patient is admitted without the prosthesis, and the
wound is covered with a partial-thickness skin graft.
Figure 2 - A: Complex wound in the anterolateral region
of the knee after adequate debridement; B:
Complex wound in the anterolateral region of the knee
covered with polyvinyl chloride (PVC) prosthesis;
C: The prosthesis is removed after six to
eight weeks, with granulation tissue filling the initial
wound.
Figure 2 - A: Complex wound in the anterolateral region
of the knee after adequate debridement; B:
Complex wound in the anterolateral region of the knee
covered with polyvinyl chloride (PVC) prosthesis;
C: The prosthesis is removed after six to
eight weeks, with granulation tissue filling the initial
wound.
After anesthesia, asepsis, and antisepsis, the granulation tissue of the
wound is carefully debrided, leaving it at the same height as the
adjacent skin.
The autologous graft is removed from the anterolateral thigh donor area
with a 32 cm Blair blade (Montserrat®), with a graft size
sufficient to cover the wound, with a thickness of 0.3 to 0.4 mm (Figure 3A).
Figure 3 - A: The autologous graft is removed from the
donor area of the anterolateral region of the thigh with a
32cm Blair blade (Montserrat®), with a graft size sufficient
to cover the wound and a thickness of 0.3 to 0.4mm;
B: The split-thickness skin graft is placed
on a sterile metal surface and subjected to multiple
parallel incisions of approximately 5-10mm; C:
The graft is cut to the shape of the granulated wound area
and sutured to the healthy wound edges. Simple sutures
(Nylon 4-0 thread) are sufficient to secure the graft to the
edge of the wound, providing a perfect fit.
Figure 3 - A: The autologous graft is removed from the
donor area of the anterolateral region of the thigh with a
32cm Blair blade (Montserrat®), with a graft size sufficient
to cover the wound and a thickness of 0.3 to 0.4mm;
B: The split-thickness skin graft is placed
on a sterile metal surface and subjected to multiple
parallel incisions of approximately 5-10mm; C:
The graft is cut to the shape of the granulated wound area
and sutured to the healthy wound edges. Simple sutures
(Nylon 4-0 thread) are sufficient to secure the graft to the
edge of the wound, providing a perfect fit.
Immediately after removing the skin, the donor area is covered with
rayon-type gauze soaked in an adrenaline solution at a concentration of
1:200,000 for 10 minutes for hemostasis, and then a dressing is made
with rayon-type gauze maintained in occlusion by sterile cotton gauze
and a bandage. The split-thickness skin graft is placed on a sterile
metal surface and subjected to multiple parallel incisions of
approximately 5-10mm. These incisions help increase the graft area and
drain secretions, preventing secretions from forming below the graft and
making it difficult to integrate with the grafted area (Figure 3B).
The graft is cut to the shape of the granulated area of the wound and
sutured at its healthy edges. Simple sutures (Nylon 4-0 thread) are
sufficient to fix the graft to the edge of the wound, providing a
perfect fit (Figure 3C). The
dressing is made of sterile gauze and crepe bandage, the first change
only after five days, and daily in the donor area. The patient is
discharged after the first dressing change in the grafted area (five
days), with the remaining dressings performed daily on an outpatient
basis. The stitches are removed after two weeks. Follow-ups occur at 15,
30, 45, 60, 90, and 180 days after split-thickness skin grafting (Figure 4).
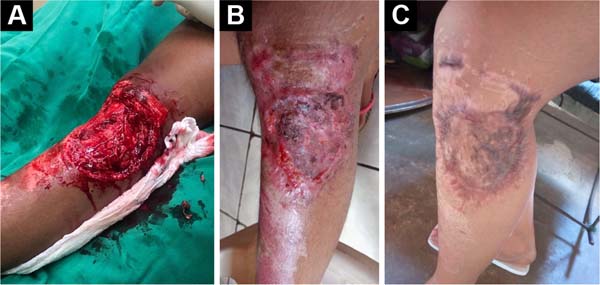
Figure 4 - A: Complex wound in the anterolateral region
of the knee; B: Complex wound in the
anterolateral region of the knee, 30 days after a
partial-thickness skin graft; C: Complex wound
in the anterolateral region of the knee, 90 days after
partial-thickness skin graft.
Figure 4 - A: Complex wound in the anterolateral region
of the knee; B: Complex wound in the
anterolateral region of the knee, 30 days after a
partial-thickness skin graft; C: Complex wound
in the anterolateral region of the knee, 90 days after
partial-thickness skin graft.
Description of technique B
The patients selected for group B underwent standard care at our institution,
performed by the dressing team, with daily changes until the wound
granulated. The dressings were changed twice a day according to the
following protocol:
- Gentle cleaning with heated 0.9% saline solution and cleaning solution with
PHMB;
- Removal of devitalized tissue through mechanical debridement;
- Cleaning the skin in the area around the lesion (perilesional) with a PHMB
cleaning solution;
- Cover with sterile gauze.
This procedure is performed until wound granulation, epithelialization, or
the plastic surgery team indicates intervention.
Data analysis
Categorical and numerical variables were tabulated and analyzed using the R
software for Mac OS X GUI 1.73 (7892 Catalina build), which provided central
tendency, percentile values, and dispersion measures.
Data normality was verified using the Shapiro-Wilk test. The homogeneity of
the variances of the groups was verified using the Levene test. To reject or
reject a null hypothesis, the comparison of the means of the groups was
performed using the t-test for independent samples. The presence of outliers
was verified through the construction of boxplots. Homoscedasticity was
tested by building a linear regression model between the variables.
Analyzes with a confidence interval of 95% and p less than 0.05 were
considered statistically significant.
RESULTS
The mean age of patients was 38.6±15.12 years, 80% male and 20% female. The
length of hospital stay, its costs, and the time until medical discharge were
shorter in group A (P<0.05). Patients submitted to technique
A had an average hospital stay of 9.9±0.7 days, while patients submitted to
technique B had an average of 37.3±2.0 days. Regarding the length of stay, the
t-test showed that there was a statistically significant difference between them
(t = -40.596, df=18, p<0.0001), with an advantage for technique A (Table 1).
Table 1 - Patient data.
| Patients |
Technique |
VAS |
Likert |
Hospitalized days |
| 1 |
A |
4 |
4 |
9 |
| 2 |
A |
5 |
5 |
10 |
| 3 |
A |
4 |
4 |
10 |
| 4 |
A |
6 |
4 |
9 |
| 5 |
A |
6 |
4 |
11 |
| 6 |
A |
5 |
3 |
10 |
| 7 |
A |
5 |
3 |
10 |
| 8 |
A |
6 |
4 |
10 |
| 9 |
A |
4 |
4 |
9 |
| 10 |
A |
5 |
4 |
11 |
| Average |
|
5.0±0.8 |
3.9±0.5 |
9.9±0.7 |
| 11 |
B |
5 |
3 |
38 |
| 12 |
B |
6 |
3 |
40 |
| 13 |
B |
4 |
two |
41 |
| 14 |
B |
5 |
two |
35 |
| 15 |
B |
4 |
two |
36 |
| 16 |
B |
5 |
3 |
38 |
| 17 |
B |
4 |
two |
37 |
| 18 |
B |
6 |
3 |
35 |
| 19 |
B |
5 |
3 |
37 |
| 20 |
B |
5 |
3 |
36 |
| Average |
B |
4.9±0.7 |
2.6±0.5 |
37.3±2.0 |
Four weeks after admission, none of the patients who underwent technique B
evolved with complete wound granulation or epithelialization. All those who
underwent technique B were approached by the plastic surgery team, with
subsequent use of flaps and/or grafts.
However, after 180 days, there was no difference in local pain between techniques
A and B (VAS 5.0±0.8 for A and VAS 4.9±0.7 for B). Regarding the VAS, the t-test
showed no statistically significant difference (t=0,28735, df=18, p=0.7771).
In
this evaluation, we had a p>0.05, confirming the null hypothesis (H0) of no
difference between the two groups (Table 1).
Neither group had complications; however, the degree of satisfaction at the end
of the follow-up (180 days) was higher in technique A, with a Likert of 3.9±0.5
for A and 2.6±0.5 for B. Regarding the Likert scale, the t-test showed that
there was a statistically significant difference between them (t=5.3571, df =18,
p<0.0001), with advantage for technique A (Table 1).
DISCUSSION
The tissue repair process of complex wounds is typically inadequate, preventing
the integrity of the integument, often requiring specialized
intervention.8
The treatment of skin wounds is dynamic, depends on the evolution of the tissue
repair phases, and is initially clinical, using mainly dressings or coverings.
Surgery is indicated when initial treatment is ineffective or
prolonged9. The
standard for reconstruction of the cutaneous tegument is the autogenous skin
graft10.
In the present article, the semi-occlusive dressing with PVC provided a
constantly humid environment through the accumulation of serous transudate,
conducive to tissue granulation. There are precedents for the medical use of
PVC
as a dressing, having already been used temporarily in burns.11, as well as after
rhytidectomy12.
Numerous devices/materials have been used in recent decades, creating an
environment conducive to tissue granulation. Figueiredo et al.4 reported satisfactory results of
the treatment of fingertip lesions, reproducible and low cost, which uses a
polypropylene prosthesis that temporarily replaces the nail and is placed over
the area of the lesion, promoting protection and stimulus for its healing by
second intention. Zook13
described using a silicone blade; Dumontier et al.14 reported using a portion
of
the X-ray film or the suture envelope itself. These materials are easily
available and adaptable, especially PVC.
Poonyakariyagorn et al.15
compared the use of a PVC dressing in partial skin graft donor areas, comparing
this material with the ready-made Op-site dressing and sterile gauze. The
authors found no difference between Op-site and PVC film regarding healing time
and pain. Both were better than gauze. The results demonstrate the usefulness
of
PVC film as a dressing for the donor area, as it promises relatively fast
healing, less pain, and is inexpensive.15.
Similarly, Meyer16 described a
modality of dressing for donor areas of partial skin grafts using PVC plastic
film. Good results have been reported without infection, and using this material
is recommended, as it has several advantages: being easily found, easy to
handle, having a very low cost, and, above all, allowing good results.16.
Many studies in the literature have shown the importance of wound coverage
(occlusive dressings) in the initial repair to avoid pain, prevent fluid loss
and protect against infection.17. Partial skin grafting is a reconstructive technique that
has many benefits, including accelerating the healing of burns, trauma, ulcers,
and other wounds, and reducing the occurrence of extensive scarring.18. In this context, there are
well-established techniques for managing the skin graft site to ensure an
adequate result and promote wound healing. However, the best results are
obtained when there is already an area of granulation tissue as a bed for the
graft. This is the great challenge for managing a complex wound: creating the
granulation bed for the graft19.
As seen in the present study (patients submitted to technique B), the simple
dressing is a time-consuming and expensive technique, especially due to the
length of hospital stay. Creating a humid ”microclimate” with PVC helped speed
up the process. The use of PVC in medical equipment has been contested in the
literature, mainly in the manufacture of catheters and serum and blood
bags.20. Phthalate
esters, primarily diethylhexyl phthalate (DEHP), represent a class of chemicals
used primarily as plasticizers for polyvinyl chloride in a wide range of
domestic and industrial applications. These phthalate esters are low-toxicity
environmental contaminants21.
However, with the evolution of the chemical industry, alternatives such as
Medical Grade PVC and DEHP-free PVC plasticizers are safer
alternatives.22. Thus,
although questionable, the use of collection bags with this type of PVC may
prove to be a relatively safe alternative.
Thus, this manuscript demonstrates a reproducible, low-cost, and effective
technique for treating complex wounds. We use a combination of inert PVC
prosthesis (easily obtained from the sterile urine collection bag), followed
by
the gold standard (skin grafting), to accelerate and optimize the healing of
complex wounds.
Limitations
Abiotic and biotic factors can degrade plastics. However, when degraded,
particles of micro and nanoplastic dimensions can be absorbed, generating a
series of factors hostile to the organism. This confirms that oxidative
stress is one of the mechanisms of cytotoxicity at the cellular level of
exposure to micro (nano) plastics. Furthermore, a study carried out by Revel
et al.23 showed that in
rats, when exposed to microplastics, it induces oxidative stress, alters
energy and lipid metabolism, and has neurotoxic effects.
In addition, due to the lack of information on the toxicology of
nanoplastics, their use is restricted to certain applications that are
directly in contact with humans, such as inclusion in cosmetics, detergents,
and foods, in order to prevent their potential toxicity and long-term side
effects24.
Thus, the major flaw and limitation of the present study is the failure to
measure the concentration of micro (nano) plastics in patients submitted to
technique A with the use of PVC. Many countries do not have clear
legislation on maximum tolerable, safe health values. Thus, in future
studies, the dosage of such polymers is of great value, further validating,
or not, the present technique.
CONCLUSION
The technique using PVC prosthesis and partial skin graft has good efficacy for
treating complex wounds, being reproducible and inexpensive.
1. Universidade Federal Rural do Semi-Árido,
Departamento de Ciências da Saúde, Mossoró, RN, Brazil
2. Hospital Tarcísio de Vasconcelos Maia,
Departamento de Ortopedia e Traumatologia, Mossoró, RN, Brazil
3. Hospital Otávio de Freitas, Departamento de
Ortopedia e Traumatologia, Recife, PE, Brazil
Corresponding author: Diego Ariel de Lima Rua
Francisco Mota, 572, Pres. Costa e Silva, Mossoró, RN, Brazil, Zip Code:
59625-900, E-mail: arieldelima.diego@gmail.com