

Original Article - Year 2023 - Volume 38 -
Bevacizumab effect on viability of free groin flaps in rats
Efeito do bevacizumabe na viabilidade de retalho inguinal livre em ratos
ABSTRACT
Introduction: Bevacizumab is among the most frequently used drugs in cancer treatment. There is evidence that some anti-angiogenic drugs reduce flap survival, but it is unclear whether this applies to Bevacizumab. We investigated the effect of Bevacizumab on the viability of free flaps in rats.
Method: The animals were randomly assigned to one of three groups. The Graft group received intravascular saline and was submitted to a full-thickness skin graft. The Flap-Saline and the Flap- BVZ groups underwent a free groin flap after receiving, respectively, intravascular saline solution or intravascular administration of Bevacizumab.
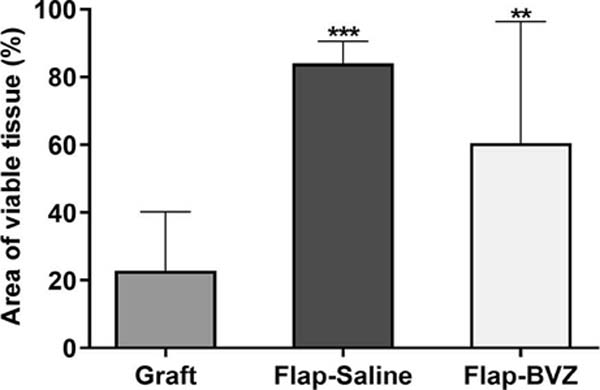
Results: The Graft group showed a lower percentage of the viable area (22.81%) relative to the Flap- Saline (83.98%; p<0.0001) and the Flap-BVZ groups (60.50%; p=0.0048). The lowest vascular pedicle patency was observed in the Flap-BVZ group, but the difference relative to the Flap-Saline was not significant (arteries, p=0.0867; veins, p=0.9999). A significant difference was observed in the occurrence of necrosis (p=0.0010), which was higher in the histological samples of the Graft (87.50%) and the Flap- BVZ (60.00%) relative to the Flap-Saline Group (0%). Inflammation occurred less frequently in the Flap-Saline (33.33%) compared to the Graft (87.5%) and Flap- BVZ group (70.00%), but the difference did not reach significance (p=0.0588). No significant differences emerged in the occurrence of hemorrhage or intraluminal thrombosis.
Conclusion: The increase in inflammation, decrease in patency and reduction of viable area, though not significant, are in line with the histological analysis and call for further research on the potential adverse effects of the drug.
Keywords: Bevacizumab; Surgical flaps; Free tissue flaps; Tissue survival; Rats; Microsurgery
RESUMO
Introdução: Bevacizumabe é um dos fármacos mais utilizados no tratamento do câncer. Existem evidências de que drogas antiangiogênicas reduzem a taxa de sobrevivência dos retalhos, porém não está claro se isso se aplica ao bevacizumabe. Investigamos o efeito de bevacizumabe na viabilidade de retalhos livres em ratos.
Método: Os animais foram randomizados em três grupos. O grupo Enxerto recebeu injeção intravenosa de soro fisiológico 0,9% (SF 0,9%) e foi submetido a uma enxertia de pele total. Os grupos Retalho-SF e Retalho-BVZ foram submetidos a retalhos inguinais livres e receberam injeções intravenosas, respectivamente, de SF 0,9% e Bevacizumabe.
Resultados: O grupo Enxerto apresentou menor percentual de área de retalho viável (22,81%) em relação ao grupo Retalho-SF (83,98%; p<0,0001) e Retalho-BVZ (60,50%; p=0,0048). Os pedículos do grupo Retalho-BVZ apresentaram menor patência, mas a diferença em relação ao grupo Retalho-SF não foi significante (artérias, p=0,0867; veias, p=0,9999). A ocorrência de necrose foi significativamente maior nos grupos Enxerto (87,50%) e Retalho-BVZ (60,00%) em relação ao grupo Retalho-SF (0%) (p=0,0010). A ocorrência de inflamação foi menor no grupo Retalho-SF (33,33%) em relação aos grupos Enxerto (87,5%) e Retalho-BVZ (70,00%), porém essa análise não atingiu significância (p=0,0588). Não houve diferenças significantes na ocorrência de hemorragia ou trombose intraluminal entre os grupos.
Conclusão: O aumento da inflamação, redução da patência e das áreas viáveis dos retalhos, apesar de não significantes, corroboram com efeitos deletérios do bevacizumabe evidenciados na análise histológica e demandam futuros estudos dos potenciais efeitos adversos da droga.
Palavras-chave: Bevacizumabe; Retalhos cirúrgicos; Retalhos de tecido biológico; Sobrevivência de tecidos; Ratos; Microcirurgia
INTRODUCTION
Angiogenesis is a process that occurs physiologically during periods of tissue growth, tissue repair, or in the reproductive cycle. This process is regulated by the balance between proangiogenic and antiangiogenic factors and results in formation of new blood vessels. Tumor growth and metastasis are highly dependent on the angiogenic process.1, 2, 3, 4 Folkmann was a pioneer in establishing, in the early 1970s, the dependency of solid tumor growth on angiogenesis, thereby encouraging further research on anti-angiogenic agents as an option for cancer treatment.5
Vascular Endothelial Growth Factor (VEGF) has been implicated as one of the major proangiogenic proteins secreted by the tumor that act upon the surrounding tissue and lead to new vessel formation. This neovascularized state favors tumor growth and its shift from a latent phase to a metastatic phase.3,4 Consequently, drugs that block angiogenesis signaling, especially anti-VEGF antibodies, are among the most used anti-angiogenic drugs adopted in treating some of the most prevalent tumors.3,4 Bevacizumab (Avastin®, Genentech) was the first FDA-approved angiogenesis inhibitor, which occurred in 2004. It was initially used to treat colorectal metastatic cancer and later associated with 5-fluoracil to treat breast, lung, ovarian, and glioblastoma cancer.3,4 It selectively binds to the VEGF-A isoform, preventing its binding to its tyrosine kinase receptors (VEGFR-1 and 2), thereby preventing the onset of the angiogenic cascade in the tumor.3,4,6
Bevacizumab (BVZ) has several side effects and has been implicated in wound-healing complications, such as dehiscence, bruising, bleeding, and infections. Some patients present these problems even after waiting for the recommended 28 days after surgery before restarting the BVZ administration.7,8
To the best of our knowledge, the effects of anti-angiogenic drugs on free flap viability in humans have not yet been investigated. These effects have been studied in animal models, but only in random flaps: DOBRYANSKY et al.9 concluded that previous endostatin (angiogenesis inhibitor) administration in mice caused a statistically significant decrease in random flap survival rates.
The number of patients diagnosed with cancer has increased worldwide. For instance, global cancer reports registered 18 million new cases and 9 million deaths related to the disease in 2017.10 The high incidence of the disease associated with the growing use of anti-angiogenic drugs increases the likelihood that cancer patients eventually need urgent reconstructive surgery or replantation. Such surgery may be required for different reasons, including cancer treatment. It is worth noting that reconstructive microsurgery revolutionized the treatment of complex lesions, some previously considered irreparable. Different reconstruction strategies have become available to treat cancer with a single surgery, transferring different types of tissues from a healthy donor area to the affected site, promoting improved functional outcomes, and decreasing the incidence of limb amputations.11
OBJECTIVE
The present study aimed to evaluate the effect of the previous administration of BVZ on the viability of free groin flaps in rats and microvascular anastomoses.
METHODS
Animals
Thirty adult male Wistar rats (207–391 g) were used in the study. This animal model offers several advantages, such as the favorable handling size of the animal and its high resistance to infection and surgical trauma. Moreover, it has been widely used in the literature,12, 13, 14, 15, and adopting it facilitates comparative analysis.
Following the legislation, the Animal Research Committee approved the study protocol, Federal University of Ceará (Protocol #126/2016).
Before undergoing surgery, the animals were kept in comfortable cages with room for four adult animals, free access to food and water, and under a 12/12-hour dark-light cycle (lights on at 5:00 a.m.) All surgical procedures were performed after intraperitoneal anesthesia injection of a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg).
All rats were individually housed after surgery and were fitted with a postoperative protector for rodents, as described by Westin and Heden16 to prevent flap cannibalization (Figure 1).
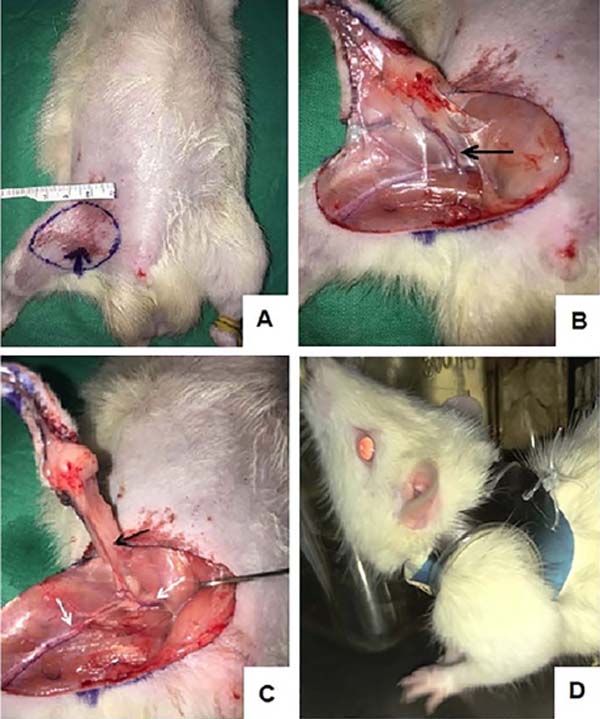
Experimental design
This is a randomized experimental study with a comparative analysis of the viability of a free flap and skin graft in the following groups, each with 10 animals. The Graft group was submitted to full-thickness skin grafting in the groin area, receiving an intravenous infusion of 0.5 mL of saline solution seven days before the procedure. The rats from the Flap-Saline group were submitted to a free groin flap after an intravenous infusion of 0.5 mL of saline solution seven days before the procedure. Finally, the animals from the BVZ-Flap group were treated with BVZ at a 5 mg/kg dose diluted in 0.5 mL of saline seven days before the free groin flap procedure.
All the treatments (BVZ or Saline solution) were administered intravenously in the lateral tail vein.
Flap Model
The surgical procedure was based on a free groin skin flap. A 3x3 cm circular area was marked on the groin and raised as a skin flap based on the right superficial caudal epigastric vessels. The vascular pedicle was dissected performing ligatures with 7-0 and 8-0 nylon sutures on all branches of the femoral vessels distal to the emergence of the superficial caudal epigastric vessels: the collateral branches (proximal caudal femoral vessels and medial proximal genicular vessels) and the distal bifurcation of the femoral vessels (popliteal and saphenous vessels) were ligated.17 The blood flow of the flap comes from the proximal segment of the femoral artery and continues directly through the superficial caudal epigastric artery (Figure 1). The superficial caudal epigastric vein toward the proximal femoral vein carries the venous drainage. The femoral artery and vein were then clamped distal to the rise of the deep femoral vessels and proximal to the take-off of the superficial caudal epigastric vessels, cut and microsurgical anastomoses were performed (10-0 nylon interrupted sutures). The flap perfusion was assessed by checking its col or the bleeding from its edges. The empty-and-refill (Acland) test was applied to the vessels to evaluate patency. If flap perfusion was judged to be compromised, the responsible anastomosis was identified through the Acland test, excised, and redone. The skin suture was performed with continuous 5-0 monofilament nylon stitches. The Graft group was submitted to the same procedure described above, but the anastomoses were not performed to compare Graft with flap survival rates. All the procedures were performed by the same surgeon (first author) through the standardized technique described above.
Flap Monitoring
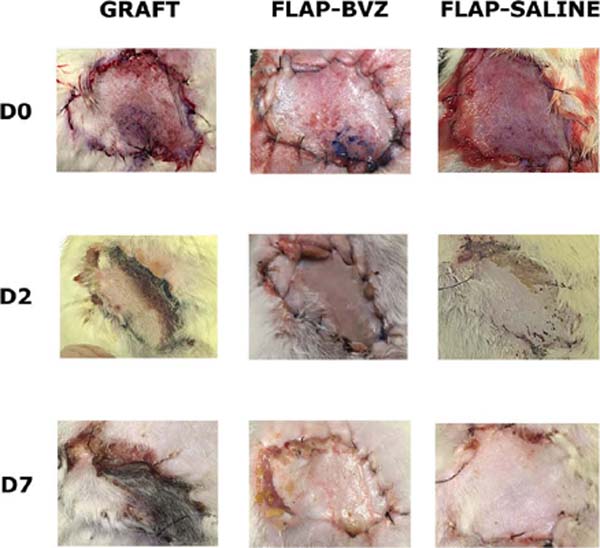
The rats were evaluated on days 0, 2, and 7 for flap or skin graft survival, color, and texture. The evaluation consisted of macroscopic analysis (performed on days 0, 2, and 7) and histological analysis (performed after the animal sacrifice on day 7).
The animals were again anesthetized on day 7, and a high-resolution digital camera (12 megapixels) was used to obtain standardized color photographs with 3024 x 4032 pixels, which allowed measurement of the necrotic and dehiscence area by using computer software (Image J version 1.52a ).18
First, the software measured the total flap area in pixels. Afterward, a manual selection of pink and white colors, considered viable tissue, was performed. Red or black areas were considered dehiscence or necrosis and excluded from the second selection. The viable tissue area was divided by the total flap area and multiplied by 100, resulting in the percentage of viable tissue. Two blinded research assistants performed the measurements separately, and the means of the two measurements were analyzed.
Furthermore, the animals from Flap-BVZ and Flap-Saline were reoperated on day 7, and their vascular pedicle was explored for patency. The empty-and-refill test (Acland test) was performed and recorded, and subsequently, the vessels were sectioned distally to the anastomosis, with the register of the presence of bleeding in the patent vessels.
Histological Analysis
The animals were sacrificed on the seventh day after surgery with a high dose of Ketamine (240 mg/kg) and Xylazine (30 mg/kg). The flap area and small segment, including the femoral artery and vein anastomoses region, were excised and fixed with 10% formaldehyde for paraffin embedding. The animals that underwent the skin grafting procedure (Graft group) had only the grafted skin area harvested. The paraffin-embedded tissues were sectioned into 5 μm slices and prepared for hematoxylin/eosin staining.
Efforts were made to position the vessels vertically in the paraffin blocks, performing axial cuts, making it possible to measure, under light microscopy, the vascular wall layer thickness and record the presence of intraluminal thrombosis. All preparations were observed, scored, and evaluated by a blinded experienced anatomopathologist.
Standardized photographs of the arterial segment slides were taken with a high-resolution digital microscope camera, and, through computer software (Image J)18, the vascular wall layers were measured to investigate intimal hyperplasia.
The skin samples were triangular, with pedicles at lower angles. Four cuts were made: pedicle zone, intermediate zone, deep dermis zone, and superficial dermis associated with the epidermis zone. The pathologist classified these sample sections, scoring necrosis and inflammation findings as absent, mild, moderate, or intense. The pathologist classified the presence of hemorrhage as either present or absent.
Statistical Analysis
Measurements of flap viability and vascular pedicle artery thickness, which were continuous variables, were initially analyzed by the Shapiro-Wilk test to verify the normality of the distribution. As this requirement was met for both variables, descriptive statistics (mean and standard deviation) were computed, and parametric methods were used.
Comparisons between the Graft, Flap-SF, and Flap-BVZ groups concerning the percentage of viable tissue area were made by performing a one-way analysis of variance (ANOVA). Whenever the ANOVA indicated significant differences, Tukey’s multiple comparisons tests were performed to verify differences between group pairs. An unpaired t-test compared the Flap-SF and Flap-BVZ groups regarding the vascular pedicle artery wall thickness.
Ordinal variables, necrosis intensity, and inflammation measurements in histological preparations were expressed as a median, interquartile range, and minimum and maximum values. Comparisons between the Graft, SF-Flap, and BVZ-Flap groups regarding these variables were made using the Kruskal-Wallis test, complemented by Dunn’s multiple comparisons tests, to verify differences between groups in pairs.
Categorical variables, such as the occurrence in the histological preparations of necrosis, inflammation, and hemorrhage in the flaps or grafts, and intraluminal thrombosis in the vascular pedicle, as well as the empty-and-refill and bleeding tests in the artery and vein of the vascular pedicle, were expressed as absolute and relative frequency. These variables were analyzed by Fisher’s exact test and the chi-square test.
All analyses employed two-tailed tests with the significance level set at p <0.05. GraphPad Prism 8.0 (GraphPad Software, San Diego, California, USA) was used to perform the statistical analysis and plot the graphics.
RESULTS
Initially, 30 animals were operated on. However, 7 animals detached the protective vest and cannibalized the flap; they were therefore removed from the study. After obtaining the Animal Research Committee’s approval, 7 new animals were randomly assigned to one Group with losses. One animal from the Graft group died in the immediate postoperative period, possibly due to anesthetic complications or bleeding. Then, of the 29 remaining animals, 10 belonged to the Flap-SF Group, 10 to the Flap-BVZ group, and 9 to the Graft group.
Macroscopic Measurement of Tissue Survival
The ANOVA showed a statistically significant difference between the groups in the percentage of tissue viability. The Graft group had a significantly lower percentage of viable tissue area than the other two groups (mean ± standard deviation: 22.81 ± 17.33%). The Flap-BVZ Group (60.50 ± 35.87%) had a lower percentage of viable tissue area relative to the Flap-Saline Group (83.98 ± 6.54%), but this difference was not statistically significant (Figure 2). Figure 3 shows the general macroscopic evolution of flap or skin graft viability of selected animals in each Group on days 0, 2, and 7.
Patency tests
The empty-and-refill test (Acland test) was positive, showing patency in a larger percentage of arteries (100%) and veins (90%) of the animals of the Flap-Saline group relative to the Flap-BVZ Group. In the latter, 60% of arteries and 80% of veins were considered patent. The difference was neither statistically significant for arteries (p = 0.0867) nor veins (p = 0.999).
The bleeding test (section of the pedicle and observation of bleeding) was coincident with the Acland test, confirming a lower percentage of patency in arteries (60%) and veins (80%) of the Flap-BVZ group relative to the Flap-Saline group (patency confirmed in 100% of arteries and 90% of veins). This difference was not significant.
Histological Analysis
Due to operational problems during the slides’ collection or preparation, it was impossible to analyze 2 out of the 29 samples (one from the Graft group; one from the Flap-Saline group). Therefore, 27 samples remained, distributed as follows: 10 from the Flap-BVZ group, 9 from the Flap-SF Group, and 8 from the Graft group.
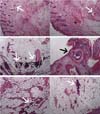
Flap skin samples were analyzed separately in four sections (epidermis and dermis, deep dermis, intermediate zone, and vascular pedicle). The anatomopathologist described the Flap -Saline Group as showing patent vessels and, in most of their samples, the absence of necrosis or inflammatory infiltrates (Figure 4).
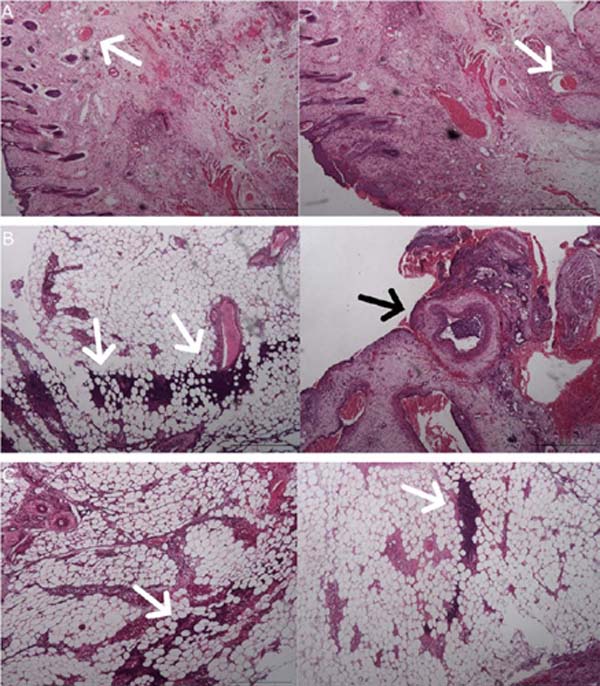
The Flap-BVZ group samples presented some thrombosed vessels and a greater number of foci of necrosis with diffuse inflammatory infiltrates (Figure 4).
The Graft group samples had a higher intensity of necrosis foci and inflammatory infiltrates than the other groups, extending throughout the depth of the different sections (Figure 4). Some vessels of the Graft group animals were also thrombosed.
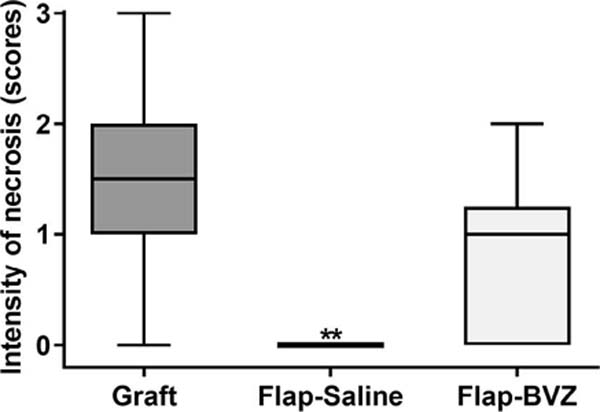
The classification of the intensity of necrosis and inflammatory infiltrates showed a significantly higher intensity of necrosis and inflammation in the Graft group compared to the Flap-Saline Group (p = 0.0015 and p = 0.0279, respectively). The Flap-BVZ Group had a higher median intensity of necrosis and inflammation than the Flap-Saline group, but this difference was insignificant (Figure 5).
Additional analyses were performed in which the histopathological parameters were evaluated considering only the presence or absence of necrosis, inflammation, and hemorrhage. Significantly higher necrosis occurred in the Graft and Flap-BVZ groups compared to the Flap-Saline Group (Table 1).
| Groups | Necrosis | Total | |
|---|---|---|---|
| Present | Absent | ||
| Graft | 7 (87,50%) | 1 (12,50%) | 8 (100,00%) |
| Flap-Saline | 0 (0,00%) | 9 (100,00%) | 9 (100,00%) |
| Flap-BVZ | 6 (60,00%) | 4 (40,00%) | 10 (100,00%) |
| Total | 13 | 14 | 27 |
Data expressed as absolute and relative (percentage) frequency of observation of 8 animals from the Graft group, 9 rats from the Flap-Saline group, and 10 animals from the Flap-BVZ Group. The chi-square test was used to investigate necrosis occurrence between the three groups. Necrosis occurrence was significantly higher in the Graft and Flap-BVZ Group than in the Flap-Saline Group (p= 0,001).
The occurrence of inflammation was lower in the Flap -Saline group than in the Graft and Flap-BVZ groups, but with marginal significance (p = 0.0588) (Table 2).
| Groups | Inflammatory infiltrates | Total | |
|---|---|---|---|
| Present | Absent | ||
| Graft | 7 (87,50%) | 1 (12,50%) | 8 (100,00%) |
| Flap-Saline | 3 (33,33%) | 6 (66,67%) | 9 (100,00%) |
| Flap-BVZ | 7 (70,00%) | 3 (30,00%) | 10 (100,00%) |
| Total | 17 | 10 | 27 |
Data expressed as absolute and relative (percentage) frequency of observation of 8 animals from the Graft group, 9 rats from the Flap-Saline group, and 10 animals from the Flap -BVZ Group. The chi-square test was used to compare the three groups regarding inflammation occurrence. There were no statistically significant differences between the three groups regarding the occurrence of inflammation. However, the observed differences can be considered marginally significant (p = 0.0588).
No significant differences were found between the three groups in the occurrence of hemorrhage.
There was no statistical difference between the Flap-Saline and Flap-BVZ groups regarding the occurrence of intraluminal thrombosis in the pedicle vessels. The measurement of the artery walls of the different groups also did not show a significant difference.
DISCUSSION
In the present study, free groin flaps were performed in rats to analyze the influence of BVZ on the viability of these flaps and their effects on the vascular pedicle, specifically in the region of the anastomoses. A significant difference was observed histologically in the occurrence of necrosis. This did not significantly reduce the percentage of macroscopic flap viable tissue or vascular pedicle patency. However, the differences between the groups in these measurements all pointed in the same direction of the histological analysis.
Multiple studies in animal models, usually using rats, mice, and rabbits, have evaluated the effect of anti-angiogenic drugs on wound healing. These drugs were initially claimed to have deleterious effects on wound closure, but some subsequent studies have concluded that partial inhibition of angiogenesis may reduce scar tissue formation, decreasing the incidence of hypertrophic scars and providing better cosmetic results.19, 20
The literature indicates several adverse effects of Bevacizumab (BVZ) on wound healing. Bruising, bleeding, dehiscence, and infection are among the most commonly reported.7, 8 However, to our knowledge, no research is evaluating the influence of BVZ on flaps. Dobryansky and colleagues9 examined the influence of an anti-angiogenic drug on random skin flaps, but endostatin was administered as an angiogenesis inhibitor.
The literature shows great divergence regarding the BVZ dosage used in rats. ACUN et al.21 concluded that a 2.5 mg/kg dose of BVZ administered intraperitoneally was sufficient to prevent the formation of intraperitoneal adhesions. Another study found this dose effective in regressing peritoneal endometriotic lesions.22 Other authors have used doses ranging between 2.5 to 10 mg/kg of BVZ intraperitoneally, repeating the dose daily or weekly.23,24 Divergences also occur when the intravenous route of administration is chosen: STEGMAYR et al.25 used higher doses, from 10 to 45 mg/kg, while THORN et al.26 employed a single dose of 1 mg/kg. A single 5 mg/kg dose of BVZ administered intravenously was adopted in this study, following SPERLING et al.27. A higher dose may significantly change some analyses of the effects of BVZ on free flaps in further research.
In the present study, we observed a lower percentage of viable tissue survival in the Graft group compared to the Flap-BVZ and the Flap-Saline groups, but the difference between the two latter groups was insignificant. The percentage of “take” of the Graft, denomination given to the area that evolves without necrosis when a skin graft is realized in humans, is about 70-100% of tissue survival.28, 29, 30 However, this high survival rate is not obtained in rat models, which have a lower “take” percentage of about 20 to 30% of graft survival. This lower rate is probably due to the difficulty of immobilizing the animals postoperatively.12, 31, 32 The finding of the Graft group, with an average of 22.81% of viable tissue, is consistent with results observed in previously mentioned studies.12, 31, 32 The mean percentage of 83.98% of the viable area in the Flap-Saline group also agrees with the literature, where the average survival rate of the free groin flap range from 75-90% of the area.33, 34, 35
In the present study, the Group treated with BVZ preoperatively had a percentage of viable tissue of 60.50%, compared to 83.98% of the Flap-Saline group. However, this difference was not statistically significant. DOBRYANSKY et al. investigated the influence of endostatin on dorsal random flap survival in mice. Twenty mice were assigned to one of two major groups: Group I, which received endostatin preoperatively, and Group II, which received saline preoperatively. The latter was considered a control group. The percentage of flap survival on the ninth postoperative day in Group I was 20%, significantly lower than the percentage observed in the control Group II (100%). The seemly deleterious effect of BVZ on the flap in this study is smaller than the effect of endostatin reported by DOBRYANSKY.9
In our study, the same patency rates were obtained in the empty-and-refill test (Acland test) and the bleeding test. Patency of 100% of arterial anastomoses and 90% of venous anastomoses were found in the Flap-Saline group. These values are consistent with those encountered in the literature, which shows that the patency in free groin flaps in rats is about 70 to 100%.13, 14, 33 The Flap-BVZ group had a lower patency rate of its vascular pedicle postoperatively than the Flap-Saline group. Both the Acland and the bleeding tests showed patency in 60% of the arteries and 80% of the veins. This reduction in patency observed in the Flap-BVZ relative to the Flap-Saline Group bordered on but was not below the significance level. Because the difference failed to reach significance, these findings should be interpreted cautiously. It cannot be excluded that Bevacizumab imposes a detrimental effect on flap vascularity, impairing perfusion and leading to necrosis. The findings regarding the occurrence of necrosis and inflammation support this possibility. However, as we stressed, caution is recommended due to the marginally significant differences, and further research is needed.
This study has limitations. First, the study required the sacrifice of Wistar rats on the seventh postoperative day. Therefore, for ethical reasons, the number of animals used should be limited to the minimum necessary for reliable statistical assessment.
The small sample size may have prevented some analyses that showed borderline differences from reaching statistical significance. Future studies with larger amounts of animals and higher doses may achieve significance in some of these analyses.
Second, we could not use other flap monitoring technologies, such as laser-Doppler flowmetry or indocyanine green fluorescence angiography, due to operational constraints. We opted for using two patency tests widely employed in studies similar to ours, either alone13, 14 or combined with other technologies.33, 36 It is noteworthy to mention that, in the latter studies, the patency tests and the other technologies showed similar results, which speak in favor of their validity.33, 36 Now that our findings have provided, as far as we know for the first time, an indication of the potential deleterious effect of BVZ, future research using additional technology is justified. Particularly relevant would be, for example, studies including histological analysis of the flap tissue with immunohistochemical staining, which could help to evaluate microvascularity.
CONCLUSION
In summary, prior administration of BVZ caused higher histological necrosis and inflammation in free groin flaps in rats, but there was no significant reduction in the percentage of flap survival and vascular pedicle patency. However, more studies are needed to confirm the observed results and estimate a safe period of BVZ withdrawal before and after performing this type of procedure.
1. Instituto Dr José Frota, Departamento de Cirurgia da Mão e Microcirurgia, Fortaleza,
CE, Brazil.
2. Universidade Federal do Ceará, Faculdade de Medicina, Unidade de Farmacologia Clínica,
Núcleo de Pesquisa e Desenvolvimento de Medicamentos, Fortaleza, CE, Brazil.
3. Universidade Federal do Ceará, Faculdade de Medicina, Fortaleza, CE, Brazil.
Corresponding author: João Mamede Instituto Dr. José Frota Rua Barão do Rio Branco, 1816, Fortaleza, CE, Brazil. Zip code: 60025-061 E-mail: joaomamed@gmail.com





 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter