

Articles - Year 2001 - Volume 16 -
A Variation of the Transverse Lumbosacral Flap in the Treatment of Sacral Pressure Sores
Variação da Técnica do Retalho Transverso Lombossacro no Tratamento das Úlceras Sacras
ABSTRACT
The author presents a modification of the lumbosacral flap for treatment of sacral pressure sores that avoids the use of skin grafts at the donor area. The modified technique was performed on thirty-six paraplegic patients, whereas only three of them had complications: two cases of epidermolysis and one with necrosis of the distal third of the flap. These results show the surgical viability of the modified technique as an additional option in the treatment of this complex pathology.
Keywords: Pressure sores; sacrum; flaps
RESUMO
O autor apresenta uma modificação da técnica do retalho lombossacro no tratamento das úlceras sacras evitando o uso de enxertia cutânea na área doadora do retalho. Utilizado em trinta e seis pacientes paraplégicos, teve apenas três casos com complicações, duas ocorrências de epidermálise e uma necrose do terço distal do retalho, demonstrando sua viabilidade cirúrgica como mais uma opção no tratamento desta patologia complexa.
Palavras-chave: Unitermos: Úlcera de decúbito; sacra; retalhos
Sacral pressure sores are the second most common pressure sores in paraplegic patients due to spinal injury. The sore appears following loss of protective sensibility: prolonged pressure over a skin area, exceeding tissue blood pressure leads to tissue necrosis. The main difficulty in the treatment of pressure sores is that these sores are the result of spinal injury, and full treatment should include the correction of the neurological lesion. The number of flaps described in the literature reflects the high incidence of recurrent pressure sores during the lives of these patients, who are mainly young adults.
The following principles must be observed in the treatment of pressure sores: avoid suture lines in the pressure areas; design flaps without interfering with future flaps, so that a sequence of surgical techniques are reserved for every region which may develop a sore; treat one ulcer at a time; excise all affected tissue; and prepare the patient for the posture limitations necessary for healing of the surgical wound.
Sacral pressure sores are treated by various randomlydesigned skin flaps. Ger(2,3) (1971) used muscle flaps of the gluteus maximus to treat sacral ulcers. Soon several authors described variations of this revolutionary surgical technique. Long term studies showed that muscle flaps became atrophic, thereby no longer serving as a cushion. Nola and Vistnes(7) (1980) studies, including the histological level, the different responses of skin and muscle to pressure. Minato et al(6) (1986) showed the superiority of skin over muscle when submitted to pressure, thereby establishing the fasciocutaneous flap as the preferred surgical treatment for pressure sores(8). In 1982, Daniel and Faibisoff(1) demonstrated that bony prominence are not covered by muscle in weight-bearing positions.
In 1978, Hill(4) described the transverse lumbosacral flap (TLF) and Lion and Rebello(5) (1993) standardized sequences of flaps, with TLF as the first flap, that maximize surgical options in the treatment of sacral sores.
In this paper a modification of the lumbosacral flap is described for treatment of sacral pressure sores that avoids the use of skin grafts at the donor area and does not interfere with other flaps.
ANATOMY
The blood supply to the skin and subcutaneous tissue of the lumbosacral region is predominantly segmental. The primary vascular flow comes from perforators of the lumbar artery, through the lumbar triangle, and from perforators of the intercostal artery, through the latissimus dorsi. Additional vascular flow comes from myocutaneous perforators of the superior gluteal artery and perforators of the sacrospinalis muscles. The subdermal vascular plexus extends uninterruptedly across the midline, with the proximal one-third showing an axial pattern and the distal two-thirds a random pattern.
PATIENT AND METHODS
Treatment of sacral pressure sores were performed, from August 1997 to November 1999 at Professor Carvalho Luz Hospital, in 36 patients, 16.6% of which were tetraplegic and 83,4% paraplegic. The average age was 25.8 years, ranging from 12 to 45 years. Ninety-one point six percent were male, 8.4% female. The spinal injuries were caused by stab wound in 13.9% of the cases, diving in shallow waters in 25 %, gunshot in 47.2%, and viral disease in 13.9%. The sores varied from 4 to 12 cm in diameter.
The treatmem is initiated by removal of debris followed by waiting the organization of the wound. During this period the patient is evaluated by a multidisciplinary team composed of a psychologist, a nutritionist, a physiotherapist, a neurologist, a urologist and a plastic surgeon. The goals are to improve the nutrition condition, to teach prevention procedures and the correct posture postoperatively, and to improve the usually depressive or rebel psychological profile. In addition, neurological follow-up, control of urinary infections and the choice of the surgical procedure in closing the ulcer is made.
During surgery the patient with Foley cateter is submitted to peridural or general anesthesia. With the patient in ventral decubirus, the spine and the vascular pedicles are delineated. The inferior limit of the flap contours the gluteus maximus flap, and it may reach the posterior iliac crest. The base of the flap should not exceed 5 cm on the vertical axis. The superior limit is curved until it meets the inferior line, with the width of the flap compatible to that of the ulcer (Fig. 2). After removal of the bursa, the flap is undermined subfascia of the subcutaneous tissue beginning with the upper edge. Undermining should not exceed 2 to 3 cm beyond the midline so that the vascular pedicles are protected. The undermining srops once the flap can be rotated. The lower edge of the flap is undermined beginning 2 to 3 cm of the middle of the rotation point and proceeding upwards after transposition of the transverse lumbosacral flap. Buried points are placed at the borders of the lesion to reduce suture tension (Figs. 3 and 4). Postoperatively antibiotics are administered for 24 hours and a suction drain is put in place for 48 hours. The patient is advised to avoid the dorsal position for 60 days.(Cases 1, 2, 3 and 4).
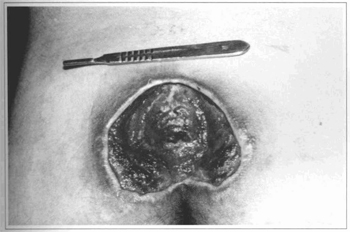
Fig. 1 - Case 1. 20 y. o. patient with sacral ulcer before surgery.
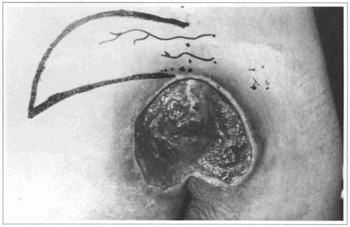
Fig. 2 - Case 1. Flap delineation.
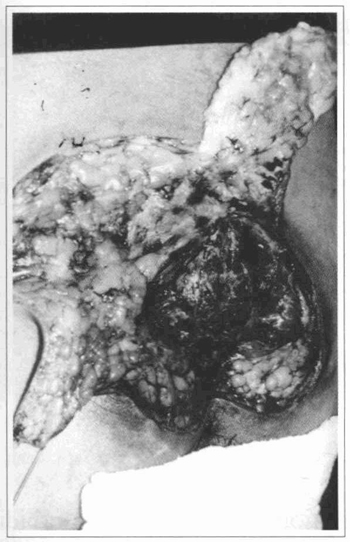
Fig. 3 - Case 1. Flap elevation.
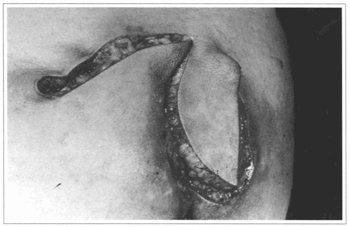
Fig. 4 - Case 1. Transposition of the flaps with anchorage points.
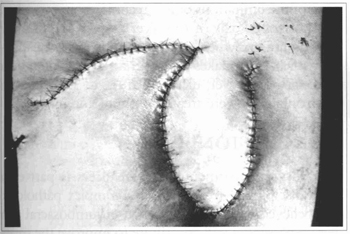
Fig. 5 - Case 1. Just after operation.
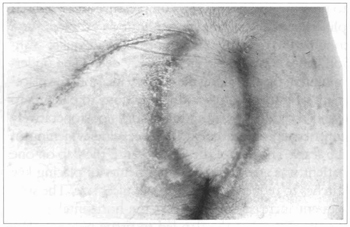
Fig. 6 - Case 1. Ninety days postoperatively.
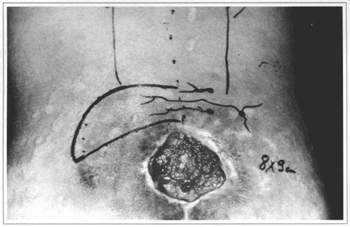
Fig. 7 - Case 2. 25 y. o. patient with sacral sore before surgery.
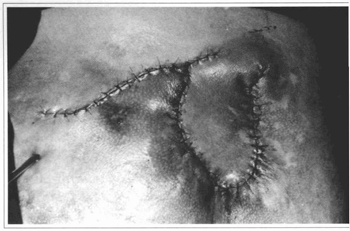
Fig. 8 - Case 2. Just after operation.
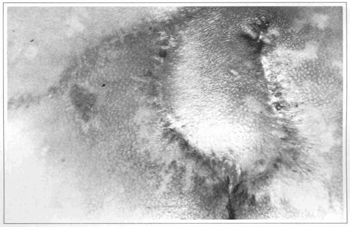
Fig. 9 - Case 2. Sixty days postoperatively.
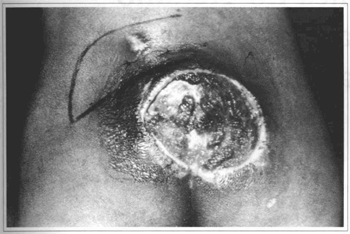
Fig. 10 - Case 3. Before surgery.
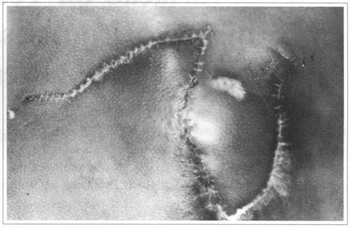
Fig. 11 - Case 3. Sixty days postoperatively.
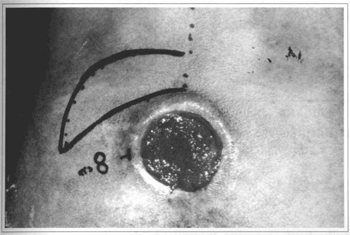
Fig. 12 - Case 4. 28 y. o. patient with 8 cm sore before surgery.
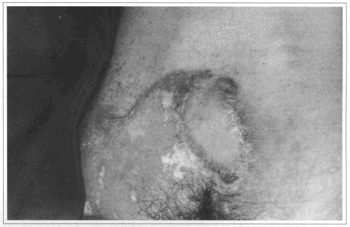
Fig. 13 - Case 4. Sixtv days postoperatively.
RESULTS
Of the 36 patients that underwent surgery, three had complications. Two had epidermolysis at the end of the lower flap toward the base of the lumbosacral flap, but the final result was not compromised. One had necrosis of the end of the flap, which healed secondarily.
DISCUSSION
The transverse lumbosacral back flap presents an excellent axial vascularization through its subdermic plexus, which allows a narrow base and the undermining of the flap under the fascia of the subcutaneous tissue without injuries ta the flap. The Z-shaped modification of the flap design allows primary healing of the danar area without complications, and shows a gaad esthetic result. Since the flap is resistant to pressure and has na scars an pressure areas, a disciplined patient may remain without recurrences for a long period of time. The flap offers an adequate cushion, since sacral ulcers are not very deep. The undermining of the flap alang the surgical incision, the placement of buried points and instructions ta patients to avoid the dorsal pasture for 60 days postoperatively contributed ta prevent dehiscences and widening of the scars. The loss af the distal part af the flap on one patient was attributed ta the difficulty of placing key stitches to reduce flap tension and ulcer size. The subsequem increase in tension at the horizontal axis of the distal third of the flap led ta tissue necrosis. The choice of the transverse lumbosacral back flap as the first surgical option is based on low morbidity of the danar area and preservation of vascularizatian of muscular ar myacutaneaus flaps of the gluteus maximus, which will be important if there is recurrence of the ulcer.
One of the limitations of this technique appears when the horizontal axis of the ulcer exceeds the primary healing limit or when an atrophic skin has low elasticity, which then makes a skin graft at the donor area necessary. However, ulcers with up to 12 cm at the horizontal axis were treated without complications.
CONCLUSION
The surgica1 treatment of sacral ulcers is part of a multidisciplinary treatment of a complex pathalagy. We believe that the modification of lumbosacral flap herein presented will contribute to improve the treatment of pressure sores due ta its technical ease, primary healing of the donor area, reduced time of the procedure and absence of scar on pressure-bearing areas. The low incidence of complications, the noninterference with future flaps and the good esthetic result are good reasons to favor its utilization.
REFERENCES
1. DANIEL RK, FAIBISOFF B. Muscle coverage of pressure points - the role of myocutaneous fiaps.Ann. Plast. Surg. 1982; 8:446-52.
2. GER R, LEVINE SA. The management of decubitus ulcers by muscle transposition: an 8-year review. Plast. Reconstr. Surg. 1976; 58:419-28.
3. GER R. The surgical management of decubitus ulcers by muscle transposition. Surg. 1971; 69:106-10.
4.HILL HL, BROWN RG, JURKIEWICZ MJ. The transverse lumbosacral back flap. Plast.Reconstr. Surg. 1978; 62(2):177-84.
5. LION PM, REBELLO C. Tratamento cirúrgica das úlceras de pressão. Rev. Col. Bras. Cirurgiões.1993; 20:332-6.
6. MINATO Y, NARA T, KASHIWA K et al. Long follow-up after coverage of ischial decubitus ulcers with myocutaneous flaps.J. Plast. Reconstr. Surg. 1986; 29:409-18.
7. NOLA GT, VISTNES LM. Differential response of skin and muscle in the experimental production of pressure sores. Plast. Reconstr. Surg. 1980; 66:728-35.
8. YAMAMOTO Y, OHURA T, SHINTOMI Y, SUGIHARA T, NOHIRA K. Superiority of the fasciocutaneous fiap in reconstruction of sacral pressure sores. Ann. Plast. Surg. 1993; 30 (2):116-21.
I - Senior Member af the Brazilian Society of Plastic Surgery.
Address for correspondence:
Géza Lászlo Ürményi, MD
R. João Garcez Froes, 136 apto. 309
40155-700 - Salvador - BA Brazil
Phone: (55 71) 235-3552


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter