

Original Article - Year 2023 - Volume 38 -
Increase in the volume of the breast implant by passing organic material into it
Aumento do volume do implante mamário por passagem de material orgânico para seu interior
ABSTRACT
Introduction: The search for an anatomical substitute for the breast, for the purpose of correcting aesthetic problems has a long history of failure until the arrival of silicone. Since the beginning of its use, in the 1960s, many complications have appeared, such as infection, rejection, rupture, in addition to silicone leakage. We did not find in the literature, however, any report on the passage of materials from the human organism to the interior of implants. The objective is to identification by infrared spectrophotometry (FTIR) and clinical analysis, reporting the passage of organic substances into breast implants without any violation of their capsule.
Methods: 1500 pairs of breast implants were analyzed, surgically removed from 1998 to 2018. Of which six were included in the study.
Results: Three materials were analyzed showing macroscopic changes in their interior, without violating the capsule. A second sample was performed on a similar implant, but without use. The third material was a sample of fatty breast tissue removed from the patient during the surgery. Materials compatible with fat, animal protein and hemoglobin were found inside the implant.
Conclusions: The change evidenced in the material inside the two implants indicates the occurrence of the passage of organic materials through an intact capsule.
Keywords: Breast; breast implants; Implant capsular contracture; Reconstructive surgical procedures; Lipids
RESUMO
Introdução: A busca de um substituto anatômico para a mama, para fins de correção de problemas estéticos, tem longa história de insucessos até a chegada do silicone. Desde o início de seu uso, na década de 1960, muitas complicações surgiram, como infecção, rejeição, rotura, além do extravasamento de silicone. Não encontramos na literatura, porém, relato algum sobre a passagem de materiais do organismo humano para o interior de implantes. O objetivo é a identificação por espectrofotometria de infravermelho (FTIR) e análise clínica, relatando a passagem de substâncias orgânicas para o interior de implantes mamários sem que os mesmos apresentem violação qualquer de sua cápsula.
Método: Foram analisados 1500 pares de implantes mamários, removidos cirurgicamente no período de 1998 a 2018. Destes, seis foram encaminhados incluídos no estudo.
Resultados: Foram analisados três materiais apresentando alterações macroscópicas em seu interior, sem que houvesse violação da cápsula. Uma segunda amostra foi realizada em implante semelhante, porém sem uso. O terceiro material foi uma amostra de tecido gorduroso mamário removido da própria paciente durante o ato cirúrgico. Foram encontrados materiais compatíveis com gordura, proteína animal e hemoglobina no interior do implante.
Conclusões: A alteração evidenciada no material do interior dos dois implantes nos indica a ocorrência de passagem de materiais orgânicos através de cápsula intacta.
Palavras-chave: Mama; Implantes de mama; Contratura capsular em implantes; Procedimentos cirúrgicos reconstrutivos; Lipídeos
INTRODUCTION
The search for an anatomical substitute for the breast to correct aesthetic problems, hypoplasia or hypotrophy, has a long history of failures until the arrival of silicone. Since the beginning of its use in the 1960s, many complications have arisen and have always been the nightmare of any surgeon, such as capsular contracture, capsule rupture, and calcification, in addition to silicone leakage into the surrounding structures, generating local manifestations1.
Several alterations were attempted in the capsule and the silicone to avoid such complications, such as the thickness and texture of the capsule, the polyurethane cover in the 1970s2, and the cohesive gel, in the 1990s3. Since then, silicone implants have increased applicability in breast augmentation and reduction surgeries, as well as correction of ptosis4,5,6, corroborated by the exponential increase in the number of studies on the subject7.
We did not find a report on the passage of materials from the body into implants in the current literature.
OBJECTIVE
The present study seeks, through identification by infrared spectrophotometry (FTIR), combined with observation and careful clinical analysis, carried out over more than 20 years, of 1500 pairs of breast implants surgically removed, to report the passage of organic substances to the interior of breast implants without showing damage, cracks or violation of any of their capsules.
METHOD
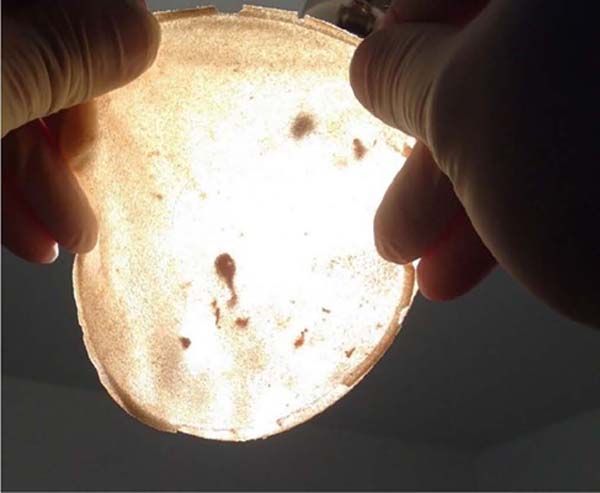
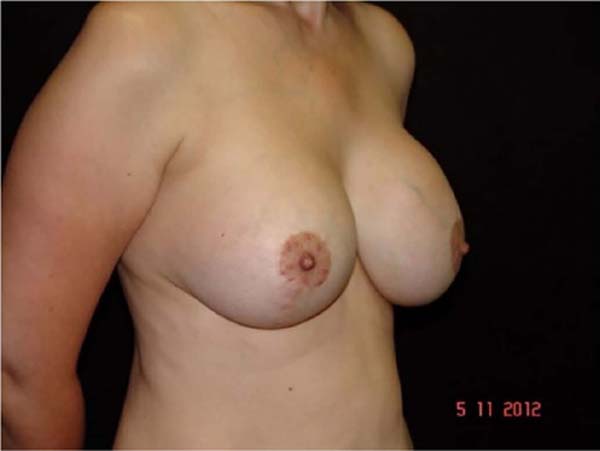
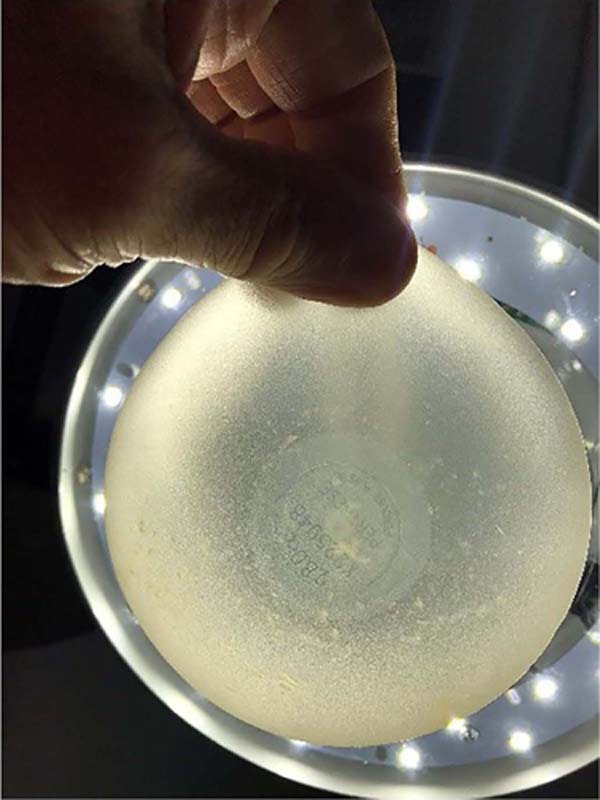
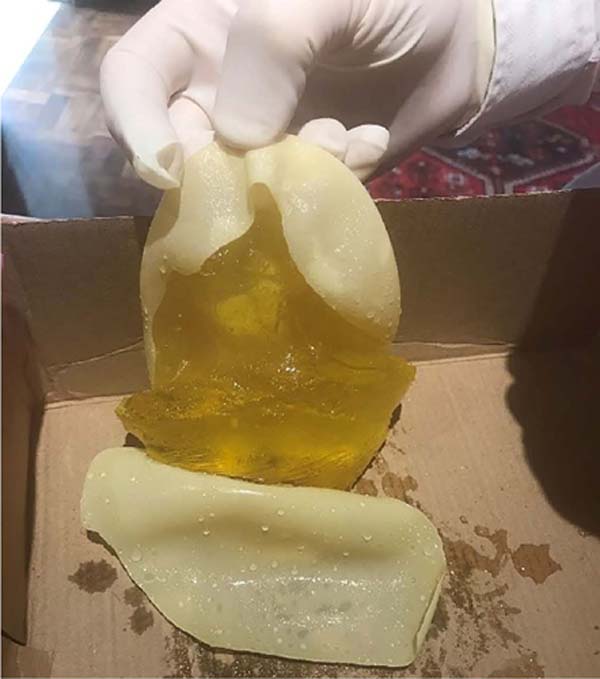
The present work clinically analyzed a sample of 1500 pairs of breast implants surgically removed from 1998 to 2018, showing changes in volume, shape, and color between units of the same pair (Figures 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10). Six pairs were sent for analysis by comparative qualitative chemical identification by infrared spectrophotometry (FTIR) before and after sample preparation by acetone solvent extraction.
Three materials were analyzed, one of which was an implant surgically explanted after years of use, showing macroscopic changes inside, without capsule violation. A second sample was performed on a similar but unused implant. The third material was a breast fat tissue sample removed from the patient during surgery. Comparative analysis was performed between all materials and the reference, and all samples were compared. There was also an evaluation of other materials found inside, different from silicone.
RESULTS
Materials compatible with fat (fatty acid ester), animal protein (hydrolyzed animal protein), and hemoglobin (protein of hemoglobin) were found inside the implant, altered after years of use, with no cracks or leaks in the external capsule. The breast fat sample was compatible with the material found inside the altered implant and the laboratory reference. Meanwhile, the only material found in the unused implant was polydimethylsiloxane, evidenced inside both samples, regardless of use, as expected.
DISCUSSION
Despite the evolution of breast implants, with changes in the gel of their content and the elastomer (wrap), complications such as capsular contracture, rupture, and microleakage persist7. The literature presents many studies of silicone migration to contiguous breast tissue and adjacent lymphatic tissue, but no publication is found on the migration of organic tissue from the patient’s body to the interior of the silicone breast prosthesis2,3.
In daily clinical practice (private clinic) dedicated to many breast surgeries, approximately 1500 cases of pair exchanges of silicone breast implants were performed, the vast majority due to capsular contracture and the silicone prosthesis rupture and aesthetic dissatisfaction of the patients.
In this 20-year series (1998 to 2018), some samples were noted that were above normal in size and weight (observation with the naked eye) and also with changes in the color of their contents, predominantly yellowish tones, but without signs of damage, cracks or violation of any of the implant casing.
In this way, without many resources at that time, the observation was carried out through transillumination, which did not bring technical analysis or veracity, but sharpened curiosity even more. The study continued with the six pairs sent for analysis by comparative qualitative chemical identification by infrared spectrophotometry (FTIR).
Materials compatible with fat (fatty acid ester), animal protein (hydrolyzed animal protein), and hemoglobin (protein of hemoglobin) were found inside the implants. In order to corroborate that the fat tissue found inside the implant could even be human and from the same patient, a small breast fat sample was resected, which served as a parameter and was compatible with the material found inside the altered implant, as well as with the laboratory reference.
This demonstrates the migration of organic components into the silicone prosthesis, proving that the possible microcracks allow the passage of content from the inside to the outside and in the opposite direction, from the outside to the inside.
CONCLUSION
The breast implant presents interaction with the organism, with the passage of substances, mainly lipids (fatty acid), animal proteins, and hemoglobin, into the interior of the implant, without damage or violation in the capsule surrounding it. This process can cause harm to the patient as it leads to inflammatory responses and increase in breast volume, often unilaterally, generating breast asymmetry, clinically confused with breast pseudo-contracture, and a possible increase in the incidence of capsular contracture, showing no difference between submuscular and subglandular implantation. Changes are usually clinically noticeable after the fourth year of surgery, appearing to be progressive.
The alteration evidenced in the material inside the two implants, which differ only in terms of use, indicates the occurrence of the passage of organic materials through the intact capsule, in a flow not yet reported in the literature, from the human body to the inorganic implant.
1. Clínica Dr. Milton Daniel, Cirurgia Plástica, Curitiba, PR, Brazil
2. Faculdade Evangélica Mackenzie do Paraná, Medicina, Curitiba, PR, Brazil
3. Hospital do Trabalhador, Cirurgia Geral, Curitiba, PR, Brazil
Corresponding author: Lincoln Graça Neto Av. Visconde de Guarapuava, 4742, Batel, Curitiba, PR, Brazil. Zip Code: 80240-010 E-mail: lgracaneto@hotmail.com


















 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter