INTRODUCTION
Soft tissue sarcomas (STS) comprise a heterogeneous group of malignant neoplasms with
different morphological patterns of the mesenchymal lineage, representing approximately
1% of malignant neoplasms in adults1. Most soft tissue sarcomas are located in the extremities, followed in order of frequency
by the abdominal cavity, retroperitoneum, trunk wall and head and neck1,2. Despite having a peak incidence in childhood, STS is more common in adulthood, especially
in people over 50 years of age1.
The staging defined by the TNM system of the International Union Against Cancer (UICC)
mainly considers the size, depth, histological grade and the presence of lymph node
metastases or distance for the composition of the stages1. The most common site of STS metastases is the lungs1,2.
The treatment of STS consists of performing a preoperative biopsy followed by resection
of the primary lesion with a safety margin above 1 cm1-3. Radiotherapy can be used
in a neoadjuvant or adjuvant manner in cases of lesions larger than 5 cm, when initially
unresectable (neoadjuvant), after resections with compromised margins (adjuvant),
recurrent lesions and in cases of high-grade lesions1,3-5.
Chemotherapy treatment is controversial and may be indicated for metastatic, recurrent,
high-grade histological and locally advanced lesions5. Reconstruction after enlarged
STS resections is a challenge, and this article aims to describe the surgical technique
for arm reconstruction using the latissimus dorsi myocutaneous flap (LDMF) in our
service.
CASE REPORT
Case 1
MFR, 61 years old, female, with a history of a progressively growing tumor on the
posterior aspect of the right arm. The patient underwent an incisional lesion biopsy
on 11/13/2020 with a histopathological result of spindle cell neoplasia with bone
neoformation and osteoclastic activity associated with multinucleated giant cells.
The positive immunohistochemical result for CD68 and SATB2 is compatible with osteosarcoma.
The MRI of the arm performed on 12/31/2020 showed a solid lesion with an epicenter
in soft tissues on the right arm’s posterior surface and a cleavage plane with the
humerus, measuring 14x10x6cm. Due to the lesion being locally advanced, after multidisciplinary
discussion, it was decided to perform neoadjuvant chemotherapy with doxorubicin followed
by radiotherapy. The tumor lesion showed significant regression after neoadjuvant
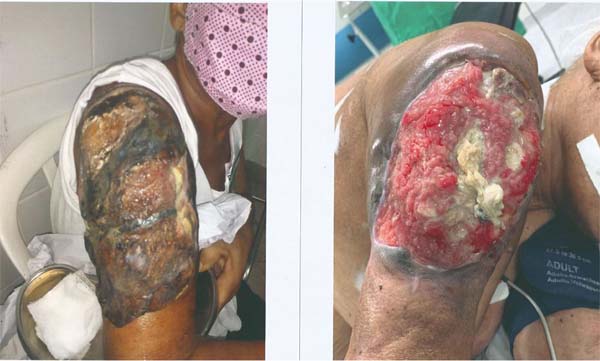
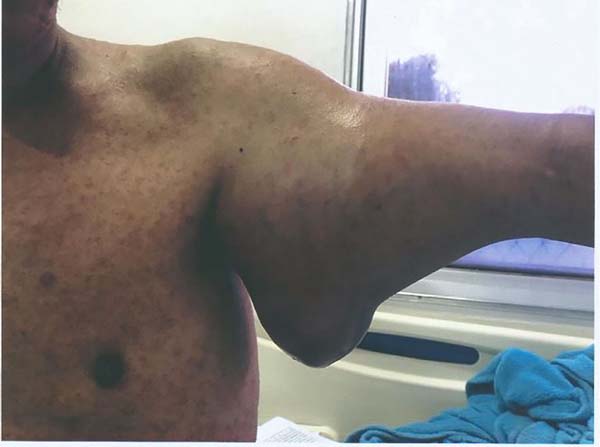
treatment (Figure 1).
Figure 1 - Tumor lesion on the posterior aspect of the right arm. Observe the lesion before (left
image) and after treatment with radiotherapy and neoadjuvant chemotherapy (right image).
Figure 1 - Tumor lesion on the posterior aspect of the right arm. Observe the lesion before (left
image) and after treatment with radiotherapy and neoadjuvant chemotherapy (right image).
On 06/07/21, the patient underwent extensive resection of the lesion, with a safety
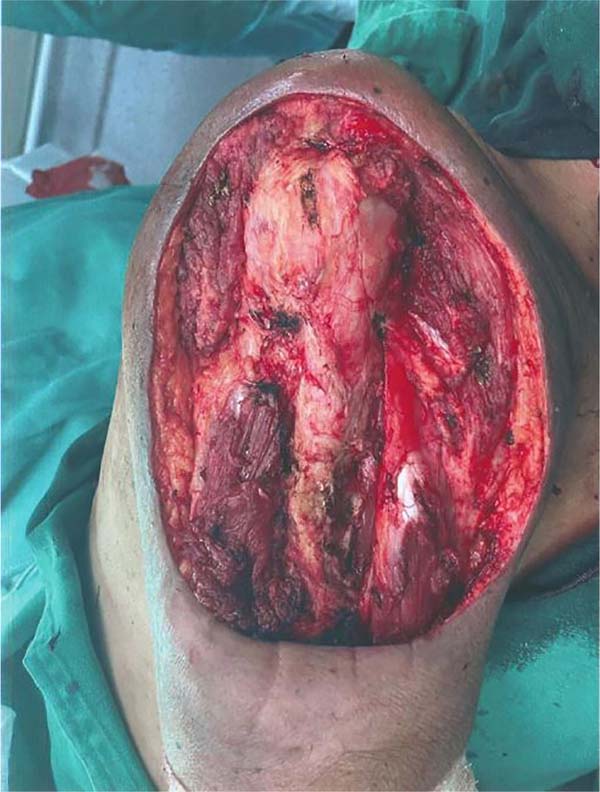
margin above 1 cm, without requiring humeral bone resection (Figure 2).
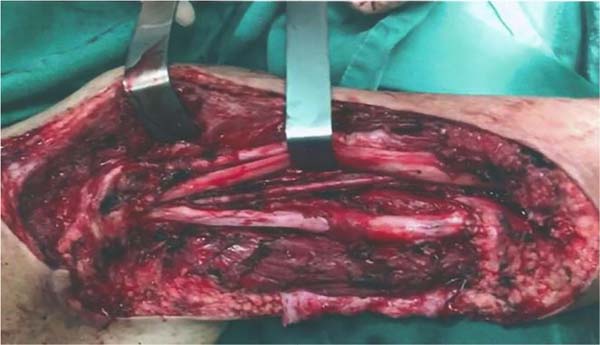
Figure 2 - Surgical bed after resectioning the lesion on the posterior aspect of the right arm.
Note the periosteum of the humerus and proximal and distal ends of the triceps brachii
muscle.
Figure 2 - Surgical bed after resectioning the lesion on the posterior aspect of the right arm.
Note the periosteum of the humerus and proximal and distal ends of the triceps brachii
muscle.
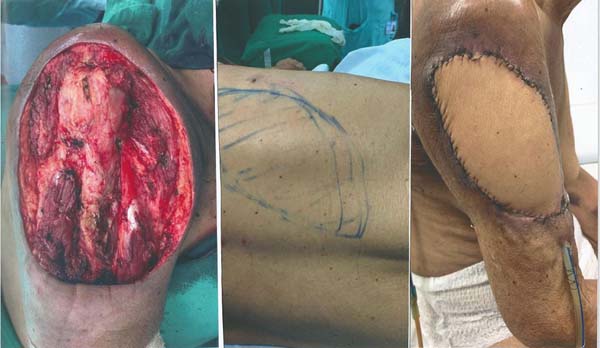
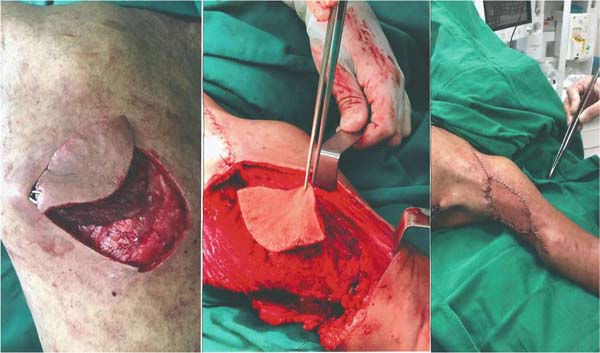
The reconstruction was performed in the same surgical procedure using the LDMF (Figure 3). The patient progressed uneventfully, and the LDMF showed no signs of ischemia.
Unfortunately, the patient evolved with lung metastases and is currently undergoing
palliative treatment with chemotherapy until the present date (01/08/2021).
Figure 3 - Closure of the resection bed using the latissimus dorsi myocutaneous flap. Observe
on the left the surgical bed after resection of the lesion with free margins, in the
center the island of skin delimited during the surgery and on the right the final
result after reconstruction.
Figure 3 - Closure of the resection bed using the latissimus dorsi myocutaneous flap. Observe
on the left the surgical bed after resection of the lesion with free margins, in the
center the island of skin delimited during the surgery and on the right the final
result after reconstruction.
Case 2
COS, 36 years old male with a history of presenting progressive growth nodulation
in the anterior aspect of the left arm for about 1 year (Figure 4). He underwent an incisional biopsy on 02/11/2019, with a result of dermatofibrosarcoma.
Immunohistochemistry was positive for CD10 and CD34 antibodies, confirming the diagnosis
of dermatofibrosarcoma. The magnetic resonance imaging of the left arm performed on
08/15/2019 showed a voluminous solid formation on the anterior face of the arm measuring
16.2x15x11.6cm, involving the vascular-nervous bundle at 180°.
Figure 4 - Injury on the anterior aspect of the left arm. The histopathological result and immunohistochemistry
were compatible with dermatofibrosarcoma.
Figure 4 - Injury on the anterior aspect of the left arm. The histopathological result and immunohistochemistry
were compatible with dermatofibrosarcoma.
The patient underwent extensive resection of the lesion, with en bloc removal of the
biceps and brachial muscles and exposure of the nerves and vessels of the anterior
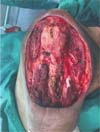
aspect of the arm (median nerve and brachial artery - Figure 5). The histopathological result was compatible with spindle cell mesenchymal neoplasia
with chondrosarcomatous dedifferentiation and free surgical margins.
Figure 5 - Note the lesion resection bed on the anterior aspect of the arm. Median nerve and
brachial artery.
Figure 5 - Note the lesion resection bed on the anterior aspect of the arm. Median nerve and
brachial artery.
The reconstruction was performed in the same surgical procedure using the LDMF (Figure 6). The patient progressed uneventfully, and the LDMF did not show any signs of ischemia.
Unfortunately, the patient evolved with lung metastases and underwent palliative chemotherapy
with doxorubicin. He died on May 7, 2020.
Figure 6 - Reconstruction of the resection bed using a latissimus dorsi myocutaneous flap. On
the left, island of skin over the latissimus dorsi muscle. Coverage and fixation of
the flap to the biceps brachii muscle (middle figure). On the right final result after
skin synthesis.
Figure 6 - Reconstruction of the resection bed using a latissimus dorsi myocutaneous flap. On
the left, island of skin over the latissimus dorsi muscle. Coverage and fixation of
the flap to the biceps brachii muscle (middle figure). On the right final result after
skin synthesis.
Description of the surgical technique
The patient is submitted to general anesthesia and positioned in lateral decubitus
with the forearm of the affected limb bandaged after rigorous antisepsis.
Resection of the primary lesion is performed following the basic oncological principles
of surgical margins greater than 1 cm and, whenever possible, without violating the
tumor pseudocapsule. Surgical margins smaller than 1 cm are allowed in cases of proximity
to the periosteum, vessels and nerves that would lead to amputation of the limb3. Surgical margin freezing, whenever available, is performed intraoperatively to avoid
compromised margins.
After resectioning the lesion in the arm with free margins, the demarcation of the
cutaneous island of the LDMF is performed with methylene blue for subsequent synthesis
of the resected skin segment on the arm. The size of this skin extension varies according
to the defect generated in the limb after resection.
The isolation of the latissimus dorsi muscle is initiated by its inferior border with
electrocautery, taking the entire available belly. The flap is released cranially,
with limits inferior to the posterosuperior iliac spine, medially to the intersection
of the thoracolumbar fascia with the trapezius muscle, laterally to the free edge
of the latissimus dorsi muscle, and superiorly to the intersection with the humerus
until the muscle insertion is identified. latissimus dorsi and the finding of the
vascular pedicle (previously demarcated). The dissection is stopped at this point,
and the flap is transported to the arm site without tension. The flap is transferred
by creating a tunnel between the dorsal region and the anterior or posterior aspect
of the arm.
Whenever possible, we leave a considerable amount of fat on the latissimus dorsi muscle
to help fill the arm, as described in breast reconstruction techniques6.
When closing the donor area, whenever possible, separate stitches of vicryl 0 were
applied to the deep planes to avoid the accumulation of seroma, and we performed closed
drainage with a 6.4 mm suction drain.
The attachment of the latissimus dorsi muscle to the surgical bed in the arm (proximal
and distal portions of the previously sectioned biceps and triceps muscles) helps
to maintain limb function. We used separate vicryl 0 stitches to attach the latissimus
dorsi muscle to the biceps and triceps brachii muscles.
The following surgical steps focus on rigorous hemostasis, washing and drainage of
the pocket with a 6.4 or 4.8 mm suction drain, synthesis of the subcutaneous tissue,
skin and, finally, dressing at the end of the procedure.
DISCUSSION
Surgery is the standard treatment for all patients with localized disease and should
be performed by an experienced surgeon, with negative (R0) and wide (1cm) margins
when possible1.
There are descriptions in the literature of the use of LDMF for upper limb reconstruction
after trauma and injuries caused by electrical burns7. In this scenario, LDMF proved to be safe and with satisfactory results for reconstruction
of the arm up to its distal third, either with a pedicled flap or a microsurgical
technique. However, the reconstruction of upper limbs after resectioning sarcomas
is rarely described in the literature8.
Behnamet al.8 published a series of 33 patients who underwent resection of sarcomas in the upper
limbs and reconstruction of the LDMF, with only two cases of partial flap necrosis.
Saba et al.9 reported a case of resection of a malignant fibrohistiocytoma in a shoulder that
underwent immediate resection and reconstruction with LDMF, with satisfactory results.
The secondary objective of maintaining limb function using the LDMF was partially
achieved in both cases. With the help of physical therapy, this result can be even
better in the long term.
It is important to emphasize that in cases of STS resection with a diameter greater
than 5 cm, adjuvant treatment with radiotherapy is indicated1,3. Thus, the closure of the primary defect with more robust tissues, as is the case
of LDMF, is an additional safety since it is not uncommon to occur radionecrosis and
wound dehiscence in cases of thin skin flaps over areas of bones and tendons10,11.
Therefore, this technique should be more widespread and applied in practice as it
allows for adequate limb reconstruction and has a low rate of complications.
CONCLUSION
Larger studies are still needed to assess the effectiveness of arm reconstruction
with LDMF since this technique is rarely described in the literature for reconstruction
in cases of STS resection. In both cases presented, LDMF was an excellent option for
reconstruction, as it allowed the primary synthesis of the lesion without complications
such as dehiscence and infection. As a secondary objective, it allowed the partial
maintenance of upper limb motor function in both cases.
REFERENCES
1. Manoel WJ, Sarmento BJQ, Silveira Júnior LP, Abreu DCB, Abreu Neto IP, Ferreira EC.
Sarcomas de partes moles: resultados do tratamento dos tumores de baixo grau. Rev
Bras Cancerol. 2008;54(1):17-24.
2. Bourcier K, Le Cesne A, Tselikas L, Adam J, Mir O, Honore C, de Baere T. Basic Knowledge
in Soft Tissue Sarcoma. Cardiovasc Intervent Radiol. 2019;42(9):1255-61. DOI: 10.1007/s00270-19-02259-w
3. Hoefkens F, Dehandschutter C, Somville J, Meijnders P, Van Gestel D. Soft tissue sarcoma
of the extremities: pending questions on surgery and radiotherapy. Radiat Oncol. 2016;11(1):136.
DOI: 10.1186/s13014-016-0668-9
4. Ray-Coquard I, Serre D, Reichardt P, Martín-Broto J, Bauer S. Options for treating
different soft tissue sarcoma subtypes. Future Oncol. 2018;14(10s):25-49. DOI: 10.2217/fon-2018-0076
5. Dujardin F, Debled M, Guillemet C, Simonet J, Hamidou H, Cambon-Michot C, et al. Diagnosis
and treatment of soft-tissue tumors]. Rev Chir Orthop Reparatrice Appar Mot. 2006;92(7):637-50.
DOI: 10.1016/s0035-1040(06)75924-4
6. Tavares-Filho JM, Franco D, Moreto L, Porchat C, Franco T. Utilização do retalho miocutâneo
de grande dorsal, com extensão adiposa, nas reconstruções mamárias: uma opção para
preenchimento do polo superior. Rev Bras Cir Plást. 2015;30(3):423-8. DOI: 10.5935/2177-1235.2015RBCP0174
7. Lazaro HA, Dupin AE, Leão CEG, Viel DO, Souza CB. Retalho de grande dorsal para reconstrução
de perda de substância por queimadura elétrica em membro superior. Rev Bras Queimaduras.
2014;13(4):265-6.
8. Behnam AB, Chen CM, Pusic AL, Mehrara BJ, Disa JJ, Athanasian EA, et al. The pedicled
latissimus dorsi flap for shoulder reconstruction after sarcoma resection. Ann Surg
Oncol. 2007;14(5):1591-5. DOI: 10.1245/s10434-006-9292-5
9. Saba SC, Shaterian A, Tokin C, Dobke MK, Wallace AM. The pedicled myocutaneous flap
as a choice reconstructive technique for immediate adjuvant brachytherapy in sarcoma
treatment. Curr Oncol. 2012;19(6):e491-5. DOI: 10.3747/co.19.1141
10. Davis AM, O’Sullivan B, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, et al; Canadian
Sarcoma Group; NCI Canada Clinical Trial Group Randomized Trial. Late radiation morbidity
following randomization to preoperative versus postoperative radiotherapy in extremity
soft tissue sarcoma. Radiother Oncol. 2005;75(1):48-53.
11. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative
versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised
trial. Lancet. 2002;359(9325):2235-41.
1. Associação Feminina de Educação e Combate ao Câncer, Vitória, ES, Brazil
Corresponding author: Leonardo Orletti Av. estudante José Júlio de Souza, 670, Apto 502, Praia de Itaparica, Vila Velha,
ES, Brazil. Zip Code: 29102-010, E-mail: leomedufes@yahoo.com.br
Article received: August 18, 2021.
Article accepted: April 7, 2022.
Conflicts of interest: none.