

Original Article - Year 2022 - Volume 37 -
Versatility and reliability of the Keystone flap in oncological reconstructions
A versatilidade e confiabilidade do retalho Keystone em reconstruções oncológicas
ABSTRACT
Introduction: The Keystone flap is an island flap with reliable vascularization and simple dissection, first described in 2003. Despite its distinct advantages, there are few scientific publications on this matter, and it is not a common option in the clinical practice of reconstructive surgery. This article aims to report the experience of a cancer referral center with Keystone flaps in oncological reconstructions.
Methods: A retrospective study was carried out data from medical records of patients who performed oncological plastic reconstruction with keystone flaps, operated by the Surgery team of the Cancer Institute of the State of São Paulo, in addition to the analysis of pre, intra and postoperative photographic records.
Results: Nine patients were identified, all with comorbidities and a mean age of 52.7. Skin defects followed after oncological resections: five in the lower extremities, three in the trunk and one in the face. The mean of the skin resected area was 52.6cm2. The reconstructions were performed under shortened surgical time. There were no postoperative complications or flap losses. The average hospital stay was 2.2 days.
Conclusion: The Keystone flap is technically simple and a reproducible option for covering wounds of different sizes and locations. Due to its reliability, simple and quick dissection, shortened hospital stay and low morbidity in the donor area, it should be considered for reconstructing cancer wounds from different locations in patients of all ages.
Keywords: Retalhos cirúrgicos; Neoplasias cutâneas; Cirurgia plástica; Oncologia cirúrgica; Procedimentos cirúrgicos reconstrutivos.
RESUMO
Introdução: O retalho Keystone é um retalho em ilha, de vascularização confiável e dissecção simples, descrito pela primeira vez em 2003. Apesar de suas vantagens, é ainda pouco citado na literatura especializada e longe de se tornar opção de escolha na prática clínica da cirurgia reconstrutiva. O objetivo deste artigo é apresentar a experiência de um serviço oncológico de alta complexidade no uso de retalhos Keystone em reconstruções.
Métodos: Um estudo retrospectivo foi desenvolvido por meio do levantamento de dados de prontuário de pacientes operados pela equipe de Cirurgia Plástica do Instituto do Câncer do Estado de São Paulo, além de análise de registros fotográficos pré, intra e pós-operatórios.
Resultados: Nove pacientes foram identificados, todos portadores de comorbidades e média de idade de 52,7 anos. Os defeitos cutâneos se seguiram após ressecções oncológicas, sendo cinco em extremidades inferiores, três em tronco e um em face. A média da área ressecada foi de 52,6cm2. As reconstruções foram realizadas sob abreviado tempo cirúrgico. Não houve complicações pós- operatórias ou perdas do retalho e o tempo de hospitalização médio foi de 2,2 dias.
Conclusão: O retalho Keystone é uma opção tecnicamente simples e reprodutível para a cobertura de ferimentos de tamanhos diversos e em localizações variadas. Devido à sua confiabilidade, dissecção simples e rápida, abreviado tempo de internação e baixa morbidade à área doadora, deve ser considerado na reconstrução de feridas oncológicas de diversas localizações, em pacientes de todas as idades.
Palavras-chave: Retalhos cirúrgicos; Neoplasias cutâneas; Cirurgia plástica; Oncologia cirúrgica; Procedimentos cirúrgicos reconstrutivos
INTRODUCTION
The Keystone flap was first described in 20031. It is an island flap based on fasciocutaneous perforators recruited from the periphery of the wound to be treated. Thus, it has the reliable vascularization of a perforating flap, combined with the simple dissection and reproducibility of a local flap2. Despite its advantages, it is still little mentioned in the specialized literature and is far from becoming an option of choice in the clinical practice of reconstructive surgery3.
OBJECTIVE
This article aims to present the experience of a highly complex oncology service in the use of Keystone flaps in reconstructions. This series of cases aims to reinforce this flap’s versatility and safety in managing defects of different volumes and locations.
METHODS
A retrospective study was developed by collecting data from the medical records of patients operated on by the Plastic Surgery team of the Cancer Institute of the State of São Paulo (ICESP) between February 2017 and January 2020. The following information
was collected: epidemiological data; histological type; comorbidities; location and size of the resected area; hospitalization time; complications. Pre, intra and postoperative photographic records were obtained.
RESULTS
A total of nine patients were treated with the Keystone flap (six women and three men), with a mean age of 52.7 years, four hypertensives, three people with diabetes and two smokers (Table 1). All defects followed after oncological resections, five in the lower extremities, three in the trunk and one in the face. Melanoma was the most frequent neoplasm. The mean resected area was 52.6cm2, with a median of 31.4cm2. In all cases, the donor area was closed primarily.
| Genre | Age | Comorbidities | Etiology | Location | Resected area | Complications | Anesthetic-surgical time | Hospitalization time | |
|---|---|---|---|---|---|---|---|---|---|
| N1 | F | 71y | SAH, DM, Depression, Obesity | Transiting metastasis of melanoma | L Leg | 19.77cm² | - | 315 min | 3 days |
| N2 | F | 73y | Breast cancer | SCC | R Ankle | 31.4cm² | - | 146 min | 2 days |
| N3 | F | 54y | Smoking, SAH, Depression | Inguinal lymphadenectomy for metastatic melanoma | R Inguinal | 21.3cm² | - | 481 min | 3 days |
| N4 | F | 76y | SAH, obesity | Infiltrative BCC | L Nasolabial sulcus | 4.35cm² | - | 238 min | 1 day |
| N5 | M | 27y | - | Sarcoma | Low back | 163.5cm² | - | 281 min | 4 days |
| N6 | M | 66y | Smoking, SAH, previous AMI | Melanoma | R Thigh | 43.96cm² | - | 280 min | 2 days |
| N7 | F | 31y | Obesity | Sarcoma | R leg | 38.46cm² | - | 255 min | 1 day |
| N8 | F | 50y | Iron deficiency anemia, arrhythmia | Sarcoma | Low back | 32.97cm² | - | 287 min | 2 days |
| N9 | M | 62y | Smoking, alcoholism | Melanoma | Back | 117.75cm² | Dog ear | 318 min | 2 days |
The anesthetic-surgical time had an average of 289 minutes. This time includes the anesthetic act, the duration of tumor resection and reconstruction by plastic surgery. The mean hospital stay was 2.2 days. The reconstructions were completed with a single surgery, except for one patient who needed to have the scar retouched due to “dog ears,” which was later performed under local anesthesia. There were no postoperative complications or flap losses. No patient was excluded from the sample.
DISCUSSION
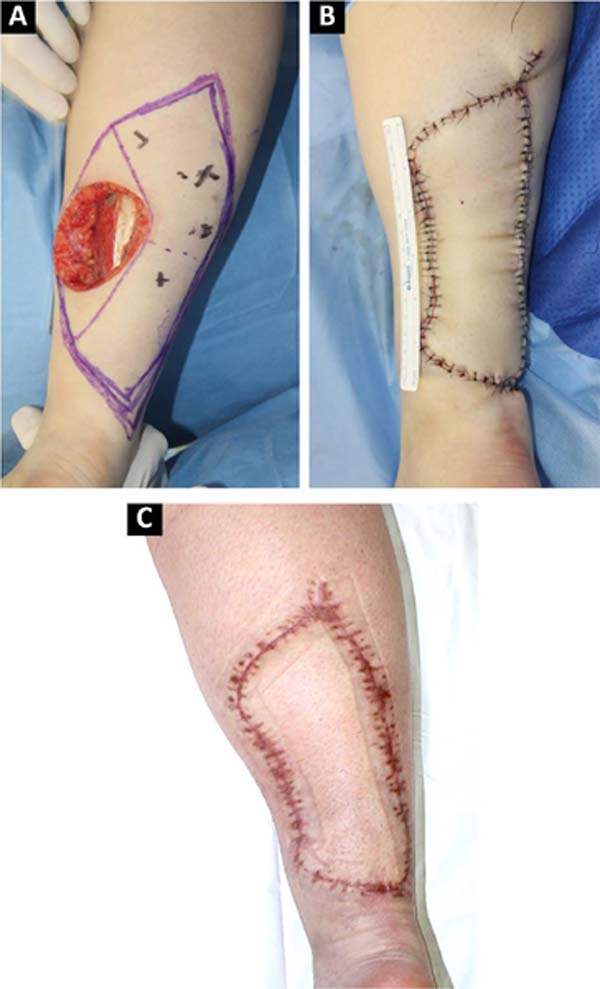
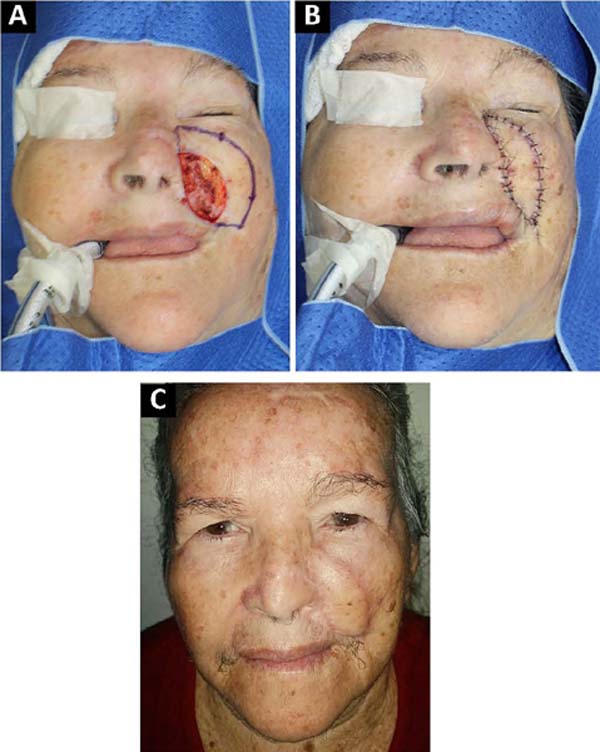
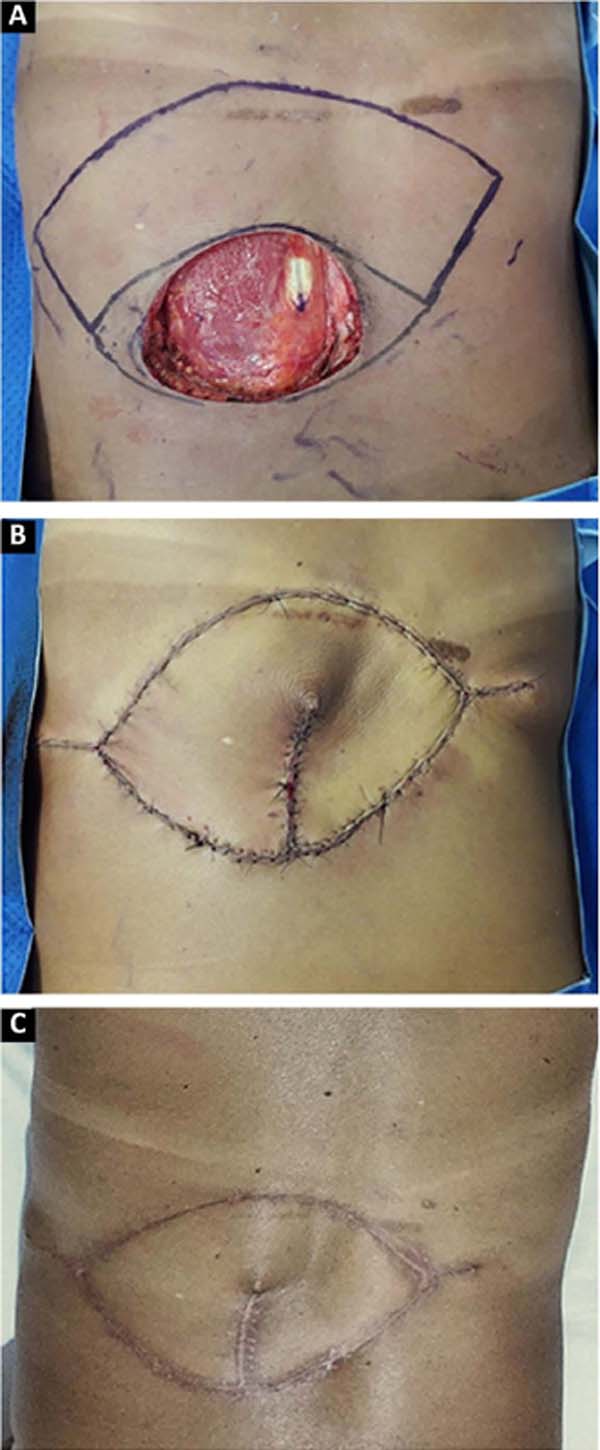
The advantages of locoregional reconstruction have already been widely discussed in the literature4. Short surgical time, stable vascularization and the satisfactory aesthetic result of coverage using tissues adjacent to the defect are some of them2. The Keystone flap combines these benefits with its versatility, and can be used in limb reconstruction2, trunk and face5, as demonstrated in our series (Figures 1, 2, 3).
Described by Behan et al. in 20031, the Keystone flap is a fasciocutaneous island flap3, 4. Its trapezoidal geometry, with a longer axis parallel to the defect, ensures the recruitment of perforating vessels in the vicinity of the wound, making its vascularization reliable. This design also allows for the advancement of tissue with little morbidity to the donor area, so the closure in at least one of the extremities will be similar to the V-Y6 flap. All donor areas were closed primarily, without major morbidities in this series.
The vascularization of this flap is proportional to the extension of the drawn skin island, as long as the area of contact with the underlying fascia is maintained, through which the perforating vessels emerge3, 4. This concept allows the manufacture of Keystone flaps of different sizes5. The rate of complications described in the literature is 4%, mainly dehiscence and partial necrosis7. In our series, the reconstructed area ranged from 4.35cm2 to 163.5cm2, without any complications with the vascularization of the flap.
The main limitation of the Keystone flap occurs when it is used in areas of inelastic skin, which restricts its advancement and makes it impossible to close the donor area without tension. Thus, it is not recommended to reconstruct the anterior face of the leg, irradiated or extensively traumatized areas8. In addition, its reliability in mucosal coverage (intraoral and intranasal) has not yet been studied3, 9. None of our patients met these restrictions.
CONCLUSION
The Keystone flap is a technically simple and reproducible option for covering wounds of different sizes and in different locations. Due to its reliability, simple and fast dissection, shortened hospital stay and low morbidity to the donor area, it should be considered in reconstructing oncological wounds of different locations in patients of all ages.
REFERENCES
1. Behan FC. The Keystone Design Perforator Island Flap in reconstructive surgery. ANZ J Surg. 2003;73(3):112-20. DOI: 10.1046/j.1445-2197.2003.02638.x PMID: 12608972 DOI: https://doi.org/10.1046/j.1445-2197.2003.02638.x
2. Rao AL, Janna RK. Keystone flap: versatile flap for reconstruction of limb defects. J Clin Diagn Res. 2015;9(3):PC05-7. DOI: 10.7860/JCDR/2015/12595.5631 PMID: 25954659 DOI: https://doi.org/10.7860/JCDR/2015/12595.5631
3. Gómez OJ, Barón OI, Peñarredonda ML. Keystone Flap: Overcoming Paradigms. Plast Reconstr Surg Glob Open. 2019;7(3):e2126. DOI: 10.1097/G0X.0000000000002126 PMID: 31044108
4. Mohan AT, Rammos CK, Akhavan AA, Martinez J, Wu PS, Moran SL, et al. Evolving Concepts of Keystone Perforator Island Flaps (KPIF): Principles of Perforator Anatomy, Design Modifications, and Extended Clinical Applications. Plast Reconstr Surg. 2016;137(6):1909-20. DOI: 10.1097/PRS.0000000000002228 PMID: 26895582
5. Pelissier P, Gardet H, Pinsolle V, Santoul M, Behan FC. The keystone design perforator island flap. Part II: clinical applications. J Plast Reconstr Aesthet Surg. 2007;60(8):888-91. DOI: 10.1016/j. bjps.2007.03.023 PMID: 17493885 DOI: https://doi.org/10.1016/j.bjps.2007.03.023
6. Shayan R, Behan FC. Re: the “keystone concept’: time for some science. ANZ J Surg. 2013;83(7-8):499-500. DOI: 10.1111/ans.12303 PMID: 24049789 DOI: https://doi.org/10.1111/ans.12303
7. Khouri JS, Egeland BM, Daily SD, Harake MS, Kwon S, Neligan PC, et al. The keystone island flap: use in large defects of the trunk and extremities in soft-tissue reconstruction. Plast Reconstr Surg. 2011;127(3):1212-21. DOI: 10.1097/PRS.0b013e318205f36f PMID: 21364423 DOI: https://doi.org/10.1097/PRS.0b013e318205f36f
8. Pauchot J, Chambert J, Remache D, Elkhyat A, Jacquet E. Geometrical analysis of the V-Y advancement flap applied to a keystone flap. J Plast Reconstr Aesthet Surg. 2012;65(8):1087-95. DOI: 10.1016/j.bjps.2012.03.004 PMID: 22512938 DOI: https://doi.org/10.1016/j.bjps.2012.03.004
9. Chen HC. Precautions in using keystone flap. J Plast Reconstr Aesthet Surg. 2010;63(4):720. DOI: 10.1016/j.bjps.2009.02.049 PMID: 19364682 DOI: https://doi.org/10.1016/j.bjps.2009.02.049
1. Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, Serviço
de Cirurgia Plástica e Queimaduras, São Paulo, SP, Brazil.
2. Instituto do Câncer do Estado de São Paulo, Serviço de Cirurgia Plástica, São Paulo,
SP, Brazil.
RDAR Analysis and/or data interpretation, Conception and design study, Conceptualization, Data Curation, Methodology, Project Administration, Writing - Original Draft Preparation, Writing -Review & Editing.
VPFP Data Curation, Investigation, Writing - Original Draft Preparation, Writing - Review & Editing.
GMC Conceptualization, Data Curation, Project Administration, Writing - Original Draft Preparation, Writing - Review & Editing.
GGT Data Curation, Methodology, Writing - Review & Editing.
FFB Conceptualization, Supervision, Writing - Review & Editing.
Corresponding author: Renan Diego Américo Ribeiro Av. Dr. Enéas de Carvalho Aguiar, 255, 8º andar, São Paulo, SP, Brazil Zip Code: 05403-900 E-mail: renanamericor@gmail.com
Article received: April 30, 2021.
Article accepted: May 18, 2021.
Conflicts of interest: none.
Institution: Universidade de São Paulo, Faculdade de Medicina, Instituto do Câncer do Estado de São Paulo e Hospital das Clínicas, São Paulo, SP, Brazil.




 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter