

Review Article - Year 2022 - Volume 37 -
Complications in orofacial harmonization procedures: a systematic review
Complicações em procedimentos de harmonização orofacial: uma revisão sistemática
ABSTRACT
Currently, facial aesthetic changes and dissatisfaction with self-image are influences that motivate the search for quick, non-surgical and less invasive cosmetic procedures. For this reason, the demand for patients looking for orofacial aesthetic procedures is increasing. The objective of this study was List the complications resulting from orofacial harmonization procedures through a systematic literature review, identifying which types of complications and areas are most affected. The following databases were searched: Medline (PubMed), SciELO, Scopus, Cochrane, Lilacs and Web of Science, from March to September 2020, selecting the studies that presented the following inclusion criteria: original studies in humans, about complications after orofacial harmonization procedures. Thirty-three studies from the literature were selected that presented reports of complications in patients after the orofacial harmonization procedure in the forehead, nose, eyes, periocular region and lips. Even the execution of less invasive facial cosmetic procedures can cause possible immediate or late complications after the procedure, especially in the periocular region and eyes. It is important to make professionals aware of this possibility beforehand.
Keywords: Esthetics; Rejuvenation; Face; Cosmetics/adverse effects; Hyaluronic acid; Skin aging.
RESUMO
Atualmente, as alterações estéticas faciais e a insatisfação com a autoimagem são influências que motivam a busca por procedimentos estéticos rápidos, não cirúrgicos e menos invasivos. Por esse motivo, a procura de pacientes que procuram por procedimentos estéticos orofaciais é crescente. O objetivo deste estudo foi elencar as complicações decorrentes dos procedimentos de harmonização orofacial por meio de uma revisão sistemática da literatura, identificando quais tipos de complicações e áreas são mais acometidas. As seguintes bases de dados foram pesquisadas: Medline (PubMed), SciELO, Scopus, Cochrane, Lilacs e Web of Science, de março a setembro de 2020, selecionando os estudos que apresentavam os seguintes critérios de inclusão: estudos originais em humanos sobre complicações após procedimentos de harmonização orofacial. Foram selecionados 33 estudos da literatura que apresentavam relatos de complicações em pacientes após o procedimento de harmonização orofacial na testa, nariz, olhos, região periocular e lábios. Mesmo a execução de procedimentos estéticos faciais menos invasivos pode acarretar possíveis complicações imediatas ou tardias após o procedimento, principalmente na região periocular e nos olhos. É importante alertar previamente os profissionais para essa possibilidade.
Palavras-chave: Estética; Rejuvenescimento; Face; Cosméticos/efeitos adversos; Ácido hialurônico; Envelhecimento da pele
INTRODUCTION
Facial aesthetic changes and dissatisfaction with self-image are increasingly present in today’s society1-3 and facial beauty parameters have a considerable influence on the population2,4. The appearance, especially of the female figure, is mentioned beauty and youth’ imposing a cosmetic standard that fights fatigue and aging5. Patients have been looking for quick, nonsurgical and less invasive procedures’ where we can find some substances that can modify facial aesthetics through the rejuvenation of signs of aging6. Thus, there is an increase in the demand for patients looking for orofacial aesthetic procedures performed by doctors and dentists7,8.
Facial applicators and fillers are the most widely used non-surgical resources for cosmetic procedures that seek to prevent or adjust the signs of aging9 through substances injected under the skin, which, although are effective and have favorable safety margins, early and late complications with various levels of severity can occur9. The increase in the performance of these dermal aesthetic procedures may be accompanied by factors that compromise patient safety and the reputation of professionals10.
In view of the consolidated medical practice in the cosmetic area and with the possibility of clinical practice by dentists on certain cosmetic factors11, the popularity and demand for patients by these professionals increases with the main objective of aesthetic facial procedures12. Although non-surgical, and with a safety margin, they can result in a strange or artificial appearance13, in addition to leading to complications and adverse effects after treatment14, causing damage to the patient15. It is of outmost importance that the professional is safe in making decisions when performing such procedures and attention to the limit of his attributions as a dentist16.
As a result of the increase in non-surgical aesthetic procedures, and aiming to alert professionals about possible damages and risks inherent to the technique, the objective of this systematic literature review was to list the complications resulting from orofacial harmonization procedures, identifying the most affected areas, contributing to conscious decision making and safer facial aesthetic procedures for the quality of life of the patient.
METHODS
Systematic review of the literature
Search strategy
This systematic review was conducted in accordance with the guidelines of the PRISMA protocol17, with the focus question: “What are the complications resulting from orofacial harmonization procedures?”. The articles were selected based on the inclusion criteria: population, intervention, comparison and outcomes (PICO) which are shown in Chart 1.
| Description | Abbreviation | Components |
|---|---|---|
| Population | P | Humans |
| Intervention | I | Orofacial harmonization |
| Comparation | C | Aesthetic procedures |
| Outcomes | O | Complications |
The bibliographic search was carried out by two reviewers from March to September 2020, without the use of filters, and the search was performed in all fields of the six selected databases: Medline (PubMed), SciELO, Scopus, Cochrane, Lilacs and Web of Science, in Portuguese, English and Spanish, using the following keywords.
English
“Botulinum toxin” and face and complications and “adverse effects”
“Hyaluronic acid” and face and complications and “adverse effects”
“Mesotherapy” and face and complications and “adverse effects”
“Collagen” and face and complications and “adverse effects”
“Lip” and face and complications and “adverse effects”
“Adipose tissue” and face and complications and “adverse effects”
“Laser” and face and complications and “adverse effects”
“Platelet-Rich Plasma” and face and complications and “adverse effects”
Portuguese
“Toxina botulínica” and face and complicações and “efeitos adversos”
“Ácido hialurônico” and face and complicações and “efeitos adversos”
“Mesoterapia” and face and complicações and “efeitos adversos”
“Colágeno” and face and complicações and “efeitos adversos”
“Lábio” and face and complicações and “efeitos adversos”
“Lipectomia” and face and complicações and “efeitos adversos”
“Laser” and face and complicações and “efeitos adversos”
“Plasma rico em plaquetas” and face and complicações and “efeitos adversos”
Spanish
“Toxinas Botulínicas” and cara and complicaciones and “efectos adversos”
“Ácido Hialurónico” and cara and complicaciones and “efectos adversos”
“Mesoterapia” and cara and complicaciones and “efectos adversos”
“Colágeno” and cara and complicaciones and “efectos adversos”
“Labio” and cara and complicaciones and “efectos adversos”
Lipectomia and cara and complicaciones and “efectos adversos”
“Rayos láser” and cara and complicaciones and “efectos adversos”
“Plasma rico en plaquetas” and cara and complicaciones and “efectos adversos”
Eligibility criteria
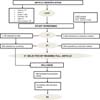
The articles were initially separated by titles, where those that did not have the topic were initially excluded. The articles selected by titles were evaluated by the reviewers through their abstracts and papers that did not meet the inclusion criteria and the repetitions found were discarded. Subsequently, a full article evaluation was carried out, where the methodological quality of each study was independently assessed by the two reviewers and the systematic selection of the studies was carried out, only those that presented the parameters of the inclusion criteria were selected for the discussion of the work.
Inclusion criteria were studies that presented complications in facial harmonization after orofacial harmonization procedures, in vivo work in humans. Exclusion criteria were literature reviews; research carried out on animals or that did not present complications resulting from orofacial harmonization procedures in the medical and dental areas.
RESULTS
Literature search
Initially 3,535 references were chosen, applying the inclusion and exclusion criteria and removing as duplicates, a final sample of 33 studies was obtained in Chart 2. A detailed description of the stages of article selection is shown in Figure 1.
| ARTICLES | TITLE | AUTHORS | YEAR | MAGAZINE |
|---|---|---|---|---|
| 1 | Complications with the Use of Botulinum Toxin Type A in Facial Rejuvenation: Report of 8 Cases19 | Ferreira MC, Salles AG, Gimenez R, Soares MFD |
2004 | Aesthetic Plastic Surgery |
| 2 | Retinal branch artery occlusion following injection of hyaluronic acid (Restylane)20 | Pedro S, Mennel S | 2006 | Clinical and Experimental Ophthalmology |
| 3 | Surgery for foreign body reactions due to injectable fillers21 | Wolfram D, Tzankov A, Piza-Katzer H |
2006 | Dermatology (Basel, Switzerland) |
| 4 | Effect of botulinum toxin type a on tear production after treatment of lateral canthal rhytids22 | Arat YO, Yen MT | 2007 | Ophthalmic Plastic and Reconstructive Surgery |
| 5 | Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel
dermal fillers: Clinical findings, long-term follow-up and review of the literature23 |
Alijotas-Reig J, Garcia GV | 2008 | Journal of the European Academy of Dermatology and Venereology |
| 6 | The risk of alar necrosis associated with dermal filler injection24 | Grunebaum LD et.al. | 2009 | Dermatologic surgery |
| 7 | Ocular damage secondary to intense pulse light therapy to the face25 | Lee WW et al. | 2011 | Ophthalmic plastic and reconstructive surgery |
| 8 | Exaggeration of wrinkles after botulinum toxin injection for forehead horizontal lines26 | Kang SM et al. | 2011 | Annals of Dermatology |
| 9 | Uncommon Foreign Body Reaction Caused byBotulinum Toxin27 | Pontes HAR et al. | 2012 | The Journal of Craniofacial Surgery |
| 10 | Facial blanching due to neurotoxins: proposed mechanisms28 | Khan TT, Herne K, Dayan SH, Woodward JA |
2013 | Dermatologic surgery |
| 11 | The use of hyaluronidase in complications caused by hyaluronic acid for volumization
of the face: a case report29 |
Neri SRNG, Addor FAZ, Parada MB, Schalka S |
2013 | Surgery cosmetic dermatology |
| 12 | Fundus artery occlusion caused by cosmetic facial injections30 | Chen Y et al. | 2014 | Chinese medical journal |
| 13 | Periorbital lipogranuloma related to fillermigration: A rare complication of facial filler31 | Eun YS, Cho SH, Lee JD, Kim HS |
2014 | Journal of Cosmetic and Laser Therapy |
| 14 | Diagnosis and management of dermal filler complications in the perioral region32 | Grippaudo FR et al. | 2014 | Journal of cosmetic and laser therapy |
| 15 | Cosmetic facial fillers and severe vision loss33 | Carle MV, Roe R, Novack R, Boyer DS |
2014 | JAMA ophthalmology |
| 16 | Clinical Outcomes of Impending Nasal Skin Necrosis Related to Nose and Nasolabial Fold Augmentation with Hyaluronic Acid Fillerso34 |
Sun ZS et al. | 2015 | Plastic and reconstructive surgery |
| 17 | Late-onset adverse reactions related to hyaluronic Acid dermal filler for aesthetic soft tissue augmentation35 | Curi MM et al. | 2015 | The Journal of craniofacial surgery |
| 18 | Cerebral angiographic findings of cosmetic facial fillerrelated ophthalmic and retinal
artery occlusion36 |
Kim YK, Jung C, Woo SJ, Park KH | 2015 | Journal of Korean Medical Science |
| 19 | Treatment of glabella skin necrosis following injection of hyaluronic acid filler using platelet-rich plasma37 | Kang BK, Kang IJ, Jeong KH, Shin MK. | 2016 | Journal of cosmetic and laser therapy |
| 20 | Retinal Branch Artery Embolization Following Hyaluronic Acid Injection: A Case Report38 | Chen W et al. | 2016 | Aesthetic surgery journal |
| 21 | Therapeutic Plasma Exchange in a rare case myasthenic crisis after Botox injection39 | Chegini A | 2017 | Atherosclerosis Supplements |
| 22 | Filler migration to the forehead due to multiple filler injections in a patient addicted to cosmetic fillers40 | Lin CH, Chiang CP, Wu BY, Gao HW | 2017 | Journal of cosmetic and laser therapy |
| 23 | Xanthelasma-Like Reaction to Filler Injection41 | Or L et al. | 2017 | Ophthalmic plastic and reconstructive surgery |
| 24 | Chronic Eyelid Edema Following Periocular Hyaluronic Acid Filler Treatment42 | Yu JTS, Peng L, Ataullah S |
2017 | Ophthalmic plastic and reconstructive surgery |
| 25 | [Clinical analysis of visual loss caused by facial cosmetic fillers injection]43 | Hu XZ et al. | 2017 | Zhonghua yan ke za zhi Chinese journal of ophthalmology |
| 26 | A Histopathologic Diagnosis of Vascular Occlusion after Injection of Hyaluronic Acid
Filler: Findings of Intravascular Foreign Body and Skin Necrosis44 |
Maruyama S | 2017 | Aesthetic Surgery Journal |
| 27 | Esotropia following botulinum toxin type A injection for facial wrinkles45 | Lee SK, Jun HJ | 2018 | Journal of cosmetic and laser therapy |
| 28 | Vascular Complications After Chin Augmentation Using Hyaluronic Acid46 |
Wang Q et al. | 2018 | Aesthetic plastic surgery |
| 29 | Complications from microfocused transcutaneous ultrasound: Case series and review of the literature47 |
Friedmann DP et. al. | 2018 | Lasers in surgery and medicine |
| 30 | Ischemic oculomotor nerve palsy due to hyaluronic acid filler injection48 | Bae IH et al. | 2018 | Journal of cosmetic dermatology |
| 31 | Horizontal animation deformity as unusual complication of neurotoxin modulation of
the gummy smile49 |
Chen G et al. | 2019 | Dermatology online journal |
| 32 | Visual loss following cosmetic facial filler injection50 | Shoughy SS | 2019 | Arquivos brasileiros de oftalmologia |
| 33 | Vascular Compromise After Soft TissueFacial Fillers: Case Report and Reviewof Current Treatment Protocols51 |
SM, Goldsmith JL, Ferneini EM | 2020 | Journal of Oral and Maxillofacial Surgery |
The cases within each study ranged from 1 to 26, with ages ranging from 22 to 74 years. The facial aesthetic procedures performed were botulinum toxin injection procedures19,22,25-28,39,45,49, filling with hyaluronic acid20,21,23,24,29,30,32-38,41-44,46,48,50,51 and other types of fillers29,41,44,48, facial treatment with intense pulsed light (IPL)25 and microned ultrasound47, there were immediate and late complications resulting from the procedures, with the eyes and the periocular region being the most affected (Table1). The methodological quality assessment core varied between 4 and 8 points, since the counted only with the positive symbol (Table 2).
| Article | n | F | M | Age | Procedure performed | Material intensity / quantity | Time elapsed from the procedure | Complications | Affected region |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 7 | 1 | 36-58 | Injection of botulinum toxin type A in the upper and lower facial third | 46 U; 10 U. | It ranged from 48 hours to 43 days. |
Headache, dry eye, progressive unilateral ptosis of the forehead and eyelid associated with diplopia, loss of control of the orbicularis oris muscle, difficulty in drinking and speaking, edema, spasms of the eye and corrugator muscles and diplopia to a greater degree. |
Facial muscles, eyes, eyelids, forehead and head |
| 2 | 1 | 0 | 1 | 48 | Filling hyaluronic acid in the glabellar area and eyelids for wrinkles | - | 1 minute | Partial visual loss in the lower half of the visual field of the right eye and occlusion of the retinal branch artery. | Eyes |
| 3 | 4 | 4 | 0 | 38-58 | Facial fillers with polymethylmethacrylate microspheres, hyaluronic acid, hyaluronic acid plus acrylic hydrogel particles and polylactic acid |
- | It ranged from 2 and 3 months to 2 years later. | Vesicular rashes on the face, granulomas, erythema, foreign body with subcutaneous and intramuscular stiffness, acute inflammation of the enlarged facial areas and also a fistula on the forehead. |
Eyelids, corner of mouth, zygomatic arch, nasolabial folds, chin, neck, cheeks and upper lip. |
| 4 | 13 | 13 | 0 | 31-58 | Botulinum toxin type A in “crow’s feet” region | 10 U for each side | 1 week | Dry eye and effects on the production of tears. | Eyes |
| 5 | 25 | - | - | - | Facial filling with hyaluronic acid (n=16) and hyaluronic acid plus hydrogels (n=9) |
- | 1-12 months | Sensitive nodules, skin tightening, systemic manifestations and other clinical signs. | Orofacial and systemic |
| 6 | 3 | 2 | 1 | 25-42 | Nose filling (rhinoplasty) with hyaluronic acid | 1 ml (n=1) | Ranged from Immediate; 12 hours after and 1 day after. | Nasal necrosis, irritation of the skin of the nose with swelling and numbness. | Nose |
| 7 | 2 | 2 | 0 | 27-36 | Facial treatment with intense pulsed light (IPL) | 560 nm with an unknown IPL unit was | 1 hour after | Eye pain, marked pupillary constriction and anterior | Eyes |
| 8 | 4 | 4 | 0 | 33-49 | Botulinum toxin in the forehead region | Less than 20 U | 1-2 weeks after | Glabellar protrusion and deep wrinkles | Forehead |
| 9 | 1 | 1 | 0 | 32 | Botulinum toxin injection to correct gingival smile | (3 BTX injections in 2 locations) |
6 months later | Nasopalatine duct cyst or lateral periodontal cyst and foreign body immersed in connective tissue of the lesion compatible with botulinum toxin. |
Maxilla and central incisors |
| 10 | 1 | 1 | 0 | 32 | Injections of botulinum toxin type A in the glabella region |
- | 2 weeks | Pale skin overlying the frontal and glabellar areas | Forehead |
| 11 | 1 | 1 | 0 | 35 | Hyaluronic acid in the region of the zygomatic arch |
18G cannula, 70mm and volume not informed |
15 days after | Pain on palpation and nodules | Zygomatic arch and infra-orbital region |
| 12 | 13 | 13 | 0 | 38-44 | Facial filling with autologous fat (n=7), hyaluronic acid (n=5) and bone collagen (n=1). Frontal (n=5), periocular (n=2), temporal (n=2) and nose (n=4) |
HA (0.6; 0.9 and 2.1 ml of material. Needle 27-30 G) and autologous fat: (20.0;2.0;5.0;12.0 and 0.9ml with a needle of 0,3; 1; 1,2 and 2mm in diameter and 23 G and 12G) |
- | Ophthalmic artery occlusion (OAO), central retinal artery occlusion (CRAO) and anterior ischemic optic neuropathy (AION). |
Eyes |
| 13 | 1 | 1 | 0 | 74 | Dermal filling with unidentified material in the forehead region | - | - | Edema and yellow discoloration of the left upper eyelid | Eyelids |
| 14 | 26 | 26 | 0 | 28-74 | Lip filling | - | - | lip volume, asymmetry, lip edema, infections, fibrosis, lumps, labial hardening, granulomatous inflammation and product migration. | Lips |
| 15 | 3 | 2 | 1 | 30-60 | Hyaluronic acid, autologous fat and microspheres of bovine collagen and polymethyl methyl acrylate on the forehead |
- | Ranged from immediately to 3 weeks after |
Visual Loss | Eyes |
| 16 | 20 | 19 | 1 | 22-52 | Hyaluronic acid in the Nose | - | 1 day after | Cutaneous necrosis (pain, erythema and edema) | Nose |
| 17 | 2 | 2 | 0 | 58-65 | Hyaluronic acid | - | 4 e 12 years later (late reaction) | Painful inflamed nodular lesions on the lip mucosa and sudden symmetrical bilateral swelling in the masseter region |
Lips and masseter |
| 18 | 7 | 7 | 0 | 24-40 | Hyaluronic acid (n=4) and autologous fat (n=3) in nose and glabella |
AH 0.2; 0.4 and 0.7 ml for autologous fat was not identified |
4 hours | Ophthalmic artery occlusion (OAO) and skin necrosis | Eyes |
| 19 | 1 | 1 | 0 | 46 | Hyaluronic acid in the forehead and nose | - | 2 days after | Redness, swelling, numerous pustules and dark regional necrosis. | Glabella, forehead and back of nose |
| 20 | 1 | 1 | 0 | 22 | Hyaluronic acid injection in the nasal dorsus | - | 10 minutes after | Nasal back and erythematous glabella accompanied by diplopia, violet discoloration, orbital pain and extrabismus in the eyes. | Glabella, nose and eyes |
| 21 | 1 | 1 | 0 | 30 | A botulim toxin injections | 3 injections without volume indication | - | Ptosis, diplopia, dysarthria, dysphagia, muscle weakness, progressive breathing difficulty and dyspnea |
Eyes and pharynx |
| 22 | 1 | 1 | 0 | 50 | Poly-lactic acid injection (PLLA) in the cheeks | - | 1 month after | Fill migration in the cheeks forming a growing nodule on the forehead | Forehead |
| 23 | 7 | 7 | 0 | 46-57 | Hyaluronic acid (n=2), synthetic calcium hydroxyapatite (n=4) and polycaprolactone microspheres (n=1) |
- | 12 months after | Reaction similar to xanthelasma on the lower eyelids that included swelling and yellow deposits on the lower eyelids |
Lower eyelids |
| 24 | 1 | 1 | 0 | 54 | Hyaluronic acid in the eyebrow region | - | 6 years after | Bilateral periorbital edema with slight blue discoloration of the skin. | Eyelids |
| 25 | 18 | 18 | 0 | 24-45 | Hyaluronic acid or autologous fat: test n=6; nose n=8; both n=4 | - | - | Visual loss (absence of light perception, ischemia at the injection site, different degrees of ptosis and examination of the fundus) |
Eyes |
| 26 | 1 | 1 | 0 | 57 | Hyaluronic acid in the glabella, forehead and nasolabial folds | 0,1mL | 2 days after | Skin necrosis, erythema, discoloration, purple, and severe pain that extends from the left glabella to the top of the forehead |
Forehead, in the parietal region |
| 27 | 1 | 1 | 0 | 36 | Botulinum toxin type A on the glabella, forehead and crow’s feet |
- | 7 days after | Esotropia partial paralysis of the eye (presented double vision) | Eyes |
| 28 | 2 | 2 | 0 | 24-42 | Hyaluronic acid (HA) | 1ml | During the procedure | Local necrosis of the chin skin, lingual paresthesia, headache and neck discomfort. | Chin and Tongue |
| 29 | 5 | 3 | 2 | 47-53 | Microfocused ultrasound (MFUS) to improve facial skin texture |
4MHz / 4.5mm, 3.0mm and 1.5mm (809 lines); 7 MHz / 4.5mm and 3.0mm (318 lines) and 4MHz / 4.5mm, 7MHz / 4.5mm and 3.0mm deep (630 lines) |
Minutes after procedure | Blisters, erosion / ulceration, skin and subcutaneous tissue and skin necrosis. | Facial skin |
| 30 | 1 | 1 | 0 | 29 | Hyaluronic acid (HA) in the nasal tip | - | During the procedure and after 3 days. | Severe pain, dizziness and blurred vision, limited extraocular movement, skin. | Periocular area and glabella |
| 31 | 1 | 1 | 0 | 28 | Botulinum toxin type A on the upper lip | one site per muscle, 2.5 units per site |
1 week after | Appearance of depressing horizontal line when smiling | Lower facial third |
| 32 | 1 | 1 | 0 | 36 | Periocular hyaluronic acid and glabella | - | - | Loss of vision in the right eye and weakness in the left arm | Eyes and arms |
| 33 | 1 | 1 | 0 | 52 | Hyaluronic acid (HA) in the puppet lines and nasolabial folds |
- | 12 hours after | Bilateral painful erythematous facial edema and palpable sensitive area with hematoma and edema | Lower third of the face and lip |
F: Female; M: Male; n: number of patients.
| Article | 1. Were patient’s demographic characteristics clearly described? |
2. Was the patient’s history clearly described and presented as a timeline? | 3. Was the current clinical condition of the patient on presentation clearly described? | 4. Were diagnostic tests or assessment methods and the results clearly described? | 5. Was the intervention(s) or treatment procedure(s) clearly described? | 6. Was the postintervention clinical condition clearly described? | 7. Were adverse events (harms) or unanticipated events identified and described? | 8. Does the case report provide takeaway lessons? |
|---|---|---|---|---|---|---|---|---|
| 119 | - | - | + | + | - | + | + | + |
| 220 | + | - | + | + | + | + | + | + |
| 321 | + | - | + | + | - | + | + | + |
| 422 | - | - | + | + | + | + | + | + |
| 523 | - | - | + | + | + | + | + | + |
| 624 | + | + | + | + | + | + | + | + |
| 725 | - | - | + | + | - | + | + | + |
| 826 | - | - | + | + | - | + | + | + |
| 927 | - | + | + | + | + | + | + | + |
| 1028 | + | - | + | + | - | + | + | + |
| 1129 | - | - | + | + | + | + | + | + |
| 1230 | + | - | + | + | + | + | + | + |
| 1331 | - | - | + | + | - | - | + | + |
| 1432 | - | - | + | + | - | + | + | + |
| 1533 | - | - | + | + | - | + | + | + |
| 1634 | + | - | + | + | - | + | + | + |
| 1735 | - | - | + | + | - | + | + | + |
| 1836 | - | - | + | + | + | + | + | + |
| 1937 | - | - | + | + | - | + | + | + |
| 2038 | + | - | + | + | - | + | + | + |
| 2139 | - | - | + | + | - | - | + | + |
| 2240 | - | - | + | + | - | + | + | + |
| 2341 | - | + | + | + | - | + | + | + |
| 2442 | + | + | + | + | - | + | + | + |
| 2543 | - | - | + | + | - | + | + | + |
| 2644 | - | - | + | + | + | + | + | + |
| 2745 | - | - | + | + | - | + | + | + |
| 2846 | - | - | + | + | - | + | + | + |
| 2947 | + | - | + | + | + | + | + | + |
| 3048 | - | - | + | + | - | + | + | + |
| 3149 | - | - | + | + | + | + | + | + |
| 3250 | - | + | + | + | - | + | + | + |
| 3351 | - | - | + | + | - | + | + | + |
DISCUSSION
The demand for an improvement in aesthetics has increased the number of facial cosmetic procedures performed52. In view of this, this study carried out an extensive analysis of possible complications after orofacial harmonization procedures so that patients and professionals are aware of these events, enabling the detection and immediate treatment.
Most of the adverse effects are non-significant and temporary, but in some exceptions they can cause a worsening of the patient’s aesthetic aspect and dissatisfaction53, causing damage and/or psychological shock in the face of frustration with their appearance, which can lead to the repair of the damage by civil liability professional54. However, even with a greater frequency of performing these services, there is still little scientific literary approach on the possible complications resulting from these procedures.
In this study, complications were reported in patients of both sexes, aged between 22 and 74; however, they were more frequent in women who performed most of the procedures22,34. These procedures are motivated by the search for rejuvenation or prevention of facial aging13. For this reason, less invasive procedures have reached greater popularity and demand9. Of these procedures, facial applicators such as botulinum toxin19,22,25-28,39,45,49 and hyaluronic acid20,21,23,24,29,30,32-38,41-44,46,48,50,51 were the most performed, probably due to the cost benefit and durability of the effect. lower eyelids and wrinkles “crow’s feet”, popularly known expression) and nose28,30,32,34,36,38,39,41,44,46,49-53,55,56. There were manifestations of complications after the procedures in the areas of the forehead, nose, eyes, periocular region and lips27-30,32-34,36-39,41,42,44-53,55,56, being the eyes and periocular region the most affected mainly with dry eye, diplopia, visual loss and ptosis1-4,7,12,13,15,18,20,21,23-25,27,32.
Consequences have been reported such as severe headache 45 lasting days, bilateral parietal headache lasting fifteen days associated with dry eye, progressive forehead ptosis, diplopia and loss of muscle control24. In the eyes, one of the most affected regions, it was possible to observe signs and symptoms such as dry eye19,22 after one month of the procedure, the symptoms remaining for four months22, eye pain with pupillary constriction and visual disturbances that resulted in permanent pupil defects even after two months after the event25, vascular problems such as occlusion of the ophthalmic artery (OAO) and anterior ophthalmic optic neuropathy (AION) have also been reported30. Visual loss occurred partially, with increased visual acuity after twenty-four hours20 and prolonged visual loss even after one year of the complication report33, the absence of light perception, esotropia45, strabismus and ischemic oculomotor nerve paralysis secondary to an occlusion vascular obstruction of an arterial branch filled with hyaluronic acid also occurred38.
Blepharoptosis48, cutaneous necrosis, edema, yellow discoloration31, reactions similar to xanthelasmas have been reported on the lower eyelids41 and a slight blue discoloration of the skin was observed even after six years of the most recent procedure42. In the regions of the glabella and forehead, where aging wrinkles are very evident, glabellar protrusion and the appearance of new very deep wrinkles occurred, in the glabellar protrusion there was a relapse with disappearance after four weeks26, pale areas, redness, swelling, severe pain, purple and skin necrosis44. There was a report of filling material applied to cheeks that migrated to the patient’s forehead, forming a nodule that was resolved only with plastic surgery45. Changes in the skin at the procedure site ranged from blisters, erosion and subcutaneous necrosis after treatment with microned ultrasound (MFUS)47, facial edema and bruising after facial applicators51.
In the nose region, especially after filling procedure using hyaluronic acid, there were cases of nasal necrosis24,34,37,38, with initial symptoms of swelling, edema, pain and erythema34, dark color37 and numbness. Cosmetic rhinomodulation procedures end up being a non-invasive cosmetic alternative to alter the nasal appearance. There were reports of two patients who recovered after appropriate treatments for necrosis of the nasal tissue, but in one case the patient had a permanent scar resulting from the complication24. In the lower third of the face, the nasopalatine duct cyst was observed, caused by the foreign body reaction, that is, the material injected with botulinum toxin32, pain, and a 3cm nodule occurred in the region of zygomatic arch after application in the region with hyaluronic acid29.
Necrosis in the chin region associated with lingual paresthesia was detected after filling with hyaluronic acid in the submental region, the ischemia of the tongue occurs by injecting material into the submental artery or its branches46. Still in the lower region of the face, the appearance of a deep horizontal line that was highlighted when the patient smiled, due to the application of botulinum toxin to correct gingival smile, the complication disappeared only after the effect of botulinum toxin ceased in three months49. A more serious complication caused breathing difficulties and dyspnea in a patient who required hospitalization and intubation for mechanical ventilation in an intensive care unit (ICU) diagnosed with myasthenia gravis, after a cosmetic procedure with botulinum toxin39. In the procedures performed on the lips, it was common to observe asymmetry, infections, fibrosis, hardening of the lips21, migration of the material used32 and painful injuries35. Systemic complications after the cosmetic procedure were also detected in a patient with no history of diseases, manifesting with fever, astralgia and arthritis23.
The present study had several relevant limitations: the nationality of the studied patients, which was not clarified; the techniques of the procedures performed; quantity and brand of material used with its concentration; the professional’s specialty was also not specified. Such information would bring greater wealth to the discussion since several professionals are able to perform such procedures, and their qualification would help to demonstrate to these classes how their care is being performed and the most common complications, in addition to detecting possible failures of execution in the procedure. The amount of material and the concentration would provide a greater explanation of why there are some complications and, consequently, try to reduce such occurrences.
Given the above, the importance of a detailed history of procedures performed by the patient is emphasized31,35, before performing the facial cosmetic procedure, as well as the complete understanding of the facial and vascular anatomy by the professional27,43,48,50,51. Which can be a contributing factor to the induction of complications related to the training and execution of procedures by the professional, with complications resulting from injection in the blood vessels, vascular lesions and occlusion, infections caused by contamination of the product and technical errors of the injection of the material57.
It is evident the importance of ensuring the keeping of good photographic documentation and always maintaining a good relationship with the patient until the complication is resolved24. It must be communicated to the patient that even though it is a simple and non-invasive procedure, complications can occur20,22,26. The professional must exercise preventive measures so that complications do not occur38, respecting professional ethics and responsibility, ensuring the patient’s health and dignity16,55, such measures avoid suffering and irreparable losses that can cause damage. Damage caused to the patient resulting from treatments can characterize civil liability55,56,58, as well as, criminal liability events can occur through bodily injury that offend the patient’s bodily integrity or health58-60. Thus, the importance of carrying out extensive studies for the detailed understanding of the possible causes and mechanisms of these events is evident in order to guarantee safer and more satisfactory aesthetic procedures for the professional and the patient.
CONCLUSIONS
It is possible to conclude that even the execution of less invasive facial cosmetic procedures can cause possible immediate or late complications after the procedure in areas of forehead, nose, lips and mainly, in the eyes and periocular region, which were the most affected with dry eyes, diplopia, visual loss and ptosis. It is important to make patients aware of this possibility beforehand. Professionals must remain alert for the immediate detection of any complications.
REFERENCES
1. Yesilbek B, Simsek S, Valério P. O impacto psicossocial da estética facial em crianças e adolescentes e a possibilidade de intervenções precoces: relato de dois casos clínicos. Rev Assoc Paul Cir Dent. 2016;70(2):192-7.
2. Gatto RCJ, Garbin AJI, Corrente JE, Garbin CAS. The relationship between oral health-related quality of life, the need for orthodontic treatment and bullying, among Brazilian teenagers. Dental Press J Orthod. 2019;24(2):73-80.
3. Garbin AJI, Wakaiama B, Saliba TA, Garbin CAS. Harmonização Orofacial e suas implicações na odontologia. Braz J Surg Clin Res. 2019;27(2):116-22.
4. Soares DM, Palmeira PTSS, Pereira VF, Santos MESM, Tassitano RM, Laureano Filho JR. Evaluation of the main criteria of facial profile aesthetics and attractiveness. Rev Bras Cir Plást. 2012;27(4):547-51.
5. Vilhena J, Medeiros S, Novaes JV. A violência da imagem: estética, feminino e contemporaneidade. Rev Mal Estar Subj. 2005;5(1):109-44.
6. de Maio M. The minimal approach: An innovation in facial cosmetic procedures. Aesthetic Plast Surg. 2004;28(5):295-300.
7. Machado MA, Flores MRP, Daruge Júnior E, Da Silva RHA. Procedimentos estéticos em Odontologia: orientações para uma prática clínica segura. Rev Dental Press Estét. 2014;11(2):90-7.
8. Pedron IG, Silva LPN. Utilização da toxina botulínica associada à cirurgia gengival ressectiva na estética dentogengival. Rev Odontol Bras Central. 2017;26(77):57-60.
9. Farolch-Prats L, Nome-Chamorro C. Facial Contouring by Using Dermal Fillers and Botulinum Toxin A: A Practical Approach. Aesthetic Plast Surg. 2019;43(3):793-802.
10. Heydenrych I, Kapoor KM, De Boulle K, Goodman G, Swift A, Kumar N, et al. 10-point plan for avoiding hyaluronic acid dermal filler-related complications during facial aesthetic procedures and algorithms for management. Clin Cosmet Investig Dermatol. 2018;11:603-11.
11. Papazian MF, Silva LM, Crepaldi AA, Crepaldi MLS, Aguiar AP. Principais aspectos dos preenchedores faciais. Rev Faipe. 2018;8(1):101-16.
12. Srivastava S, Kharbanda S, Pal US, Shah V. Applications of botulinum toxin in dentistry: A comprehensive review. Natl J Maxillofac Surg. 2015;6(2):152-9.
13. Fitzgerald R, Carqueville J, Yang PT. An approach to structural facial rejuvenation with fillers in women. Int J Womens Dermatol. 2018;5(1):52-67.
14. Zagui MRB, Matayoshi S, Moura FC. Efeitos adversos associados à aplicação de toxina botulínica na face: revisão sistemática com meta-análise. Arq Bras Oftalmol. 2008;71(6):894-901.
15. Brasil. Ministério da Saúde. Documento de referência para o Programa Nacional de Segurança do Paciente / Ministério da Saúde; Fundação Oswaldo Cruz; Agência Nacional de Vigilância Sanitária. [Internet]. Brasília: Ministério da Saúde; 2014. 40 p. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/documento_referencia_programa_nacional_seguranca.pdf
16. Conselho Federal de Odontologia de São Paulo. Resolução N°118/2012, 11 de maio de 2012. Código de ética odontológico. [Internet]. São Paulo: Conselho Federal de Odontologia; 2012. Available from: http://www.crosp.org.br/uploads/etica/6ac4d2e1ab8cf02b189238519d74fd45.pdf
17. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
18. The Joanna Briggs Institute. 2017. Critical Appraisal Checklist for Case Reports. [Internet]. [cited 2020 Mar 10]. Available from: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Case_Reports2017_0.pdf
19. Ferreira MC, Salles AG, Gimenez R, Soares MF. Complications with the use of botulinum toxin type a in facial rejuvenation: report of 8 cases. Aesthetic Plast Surg. 2004;28(6):441-4.
20. Peter S, Mennel S. Retinal branch artery occlusion following injection of hyaluronic acid (Restylane). Clin Exp Ophthalmol. 2006;34(4):363-4.
21. Wolfram D, Tzankov A, Piza-Katzer H. Surgery for foreign body reactions due to injectable fillers. Dermatology. 2006;213(4):300-4.
22. Arat YO, Yen MT. Effect of botulinum toxin type a on tear production after treatment of lateral canthal rhytids. Ophthalmic Plast Reconstr Surg. 2007;23(1):22-4.
23. Alijotas-Reig J, Garcia-Gimenez V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long-term follow-up and review of the literature. J Eur Acad Dermatol Venereol. 2008;22(2):150-61.
24. Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L. The risk of alar necrosis associated with dermal filler injection. Dermatol Surg. 2009;35 Suppl 2:1635-40.
25. Lee WW, Murdock J, Albini TA, O’brien TP, Levine ML. Ocular damage secondary to intense pulse light therapy to the face. Ophthalmic Plast Reconstr Surg. 2011;27(4):263-5.
26. Kang SM, Feneran A, Kim JK, Park O, Kim JE, Won CH, et al. Exaggeration of wrinkles after botulinum toxin injection for forehead horizontal lines. Ann Dermatol. 2011;23(2):217-21.
27. Pontes HA, Pontes FS, de Oliveira GF, de Almeida HA, Guimarães DM, Cavallero FC. Uncommon foreign body reaction caused by botulinum toxin. J Craniofac Surg. 2012;23(4):e303-5.
28. Khan TT, Herne K, Dayan SH, Woodward JA. Facial blanching due to neurotoxins: proposed mechanisms. Dermatol Surg. 2013;39(1 Pt 1):24-9.
29. Neri SRNG, Addor FAS, Parada MB, Schalka S. The use of hyaluronidase in complications caused by hyaluronic acid for volumization of the face: a case report. Surg Cosmet Dermatol. 2013;5(4):364-6.
30. Chen Y, Wang W, Li J, Yu Y, Li L, Lu N. Fundus artery occlusion caused by cosmetic facial injections. Chin Med J (Engl). 2014;127(8):1434-7.
31. Eun YS, Cho SH, Lee JD, Kim HS. Periorbital lipogranuloma related to filler migration: a rare complication of facial fillers. J Cosmet Laser Ther. 2014;16(3):149-50.
32. Grippaudo FR, Di Girolamo M, Mattei M, Pucci E, Grippaudo C. Diagnosis and management of dermal filler complications in the perioral region. J Cosmet Laser Ther. 2014;16(5):246-52.
33. Carle MV, Roe R, Novack R, Boyer DS. Cosmetic facial fillers and severe vision loss. JAMA Ophthalmol. 2014;132(5):637-9.
34. Sun ZS, Zhu GZ, Wang HB, Xu X, Cai B, Zeng L, et al. Clinical Outcomes of Impending Nasal Skin Necrosis Related to Nose and Nasolabial Fold Augmentation with Hyaluronic Acid Fillers. Plast Reconstr Surg. 2015;136(4):434e-41e.
35. Curi MM, Cardoso CL, Curra C, Koga D, Benini MB. Lateonset adverse reactions related to hyaluronic Acid dermal filler for aesthetic soft tissue augmentation. J Craniofac Surg. 2015;26(3):782-4.
36. Kim YK, Jung C, Woo SJ, Park KH. Cerebral Angiographic Findings of Cosmetic Facial Filler-related Ophthalmic and Retinal Artery Occlusion. J Korean Med Sci. 2015;30(12):1847-55.
37. Kang BK, Kang IJ, Jeong KH, Shin MK. Treatment of glabella skin necrosis following injection of hyaluronic acid filler using platelet-rich plasma. J Cosmet Laser Ther. 2016;18(2):111-2.
38. Chen W, Wu L, Jian XL, Zhang B, Li JY, Qin XL, et al. Retinal Branch Artery Embolization Following Hyaluronic Acid Injection: A Case Report. Aesthet Surg J. 2016;36(7):NP219-24.
39. Chegini A. Therapeutic Plasma Exchange in a rare case myasthenic crisis after Botox injection. Atheroscler Suppl. 2017;30:283-5.
40. Lin CH, Chiang CP, Wu BY, Gao HW. Filler migration to the forehead due to multiple filler injections in a patient addicted to cosmetic fillers. J Cosmet Laser Ther. 2017;19(2):124-6.
41. Or L, Eviatar JA, Massry GG, Bernardini FP, Hartstein ME. Xanthelasma-Like Reaction to Filler Injection. Ophthalmic Plast Reconstr Surg. 2017;33(4):244-7.
42. Yu JTS, Peng L, Ataullah S. Chronic Eyelid Edema Following Periocular Hyaluronic Acid Filler Treatment. Ophthalmic Plast Reconstr Surg. 2017;33(6):e139-40.
43. Hu XZ, Chen SQ, Zhang Q, Wu PS, Lu W. Clinical analysis of visual loss caused by facial cosmetic fillers injection. Zhonghua Yan Ke Za Zhi. 2017;53(8):594-8.
44. Maruyama S. A Histopathologic Diagnosis of Vascular Occlusion After Injection of Hyaluronic Acid Filler: Findings of Intravascular Foreign Body and Skin Necrosis. Aesthet Surg J. 2017;37(9):NP102-8.
45. Lee SK, Jun HJ. Esotropia following botulinum toxin type A injection for facial wrinkles. J Cosmet Laser Ther. 2018;20(1):50-1.
46. Wang Q, Zhao Y, Li H, Li P, Wang J. Vascular Complications After Chin Augmentation Using Hyaluronic Acid. Aesthetic Plast Surg. 2018;42(2):553-9.
47. Friedmann DP, Bourgeois GP, Chan HHL, Zedlitz AC, Butterwick KJ. Complications from microfocused transcutaneous ultrasound: Case series and review of the literature. Lasers Surg Med. 2018;50(1):13-9.
48. Bae IH, Kim MS, Choi H, Na CH, Shin BS. Ischemic oculomotor nerve palsy due to hyaluronic acid filler injection. J Cosmet Dermatol. 2018;17(6):1016-8.
49. Chen G, Oranges CM, Giordano S, Huang R, Wang W. Horizontal animation deformity as unusual complication of neurotoxin modulation of the gummy smile. Dermatol Online J. 2019;25(8):13030/qt49s9h9zh.
50. Shoughy SS. Visual loss following cosmetic facial filler injection. Arq Bras Oftalmol. 2019;82(6):511-3.
51. Halepas S, Peters SM, Goldsmith JL, Ferneini EM. Vascular Compromise After Soft Tissue Facial Fillers: Case Report and Review of Current Treatment Protocols. J Oral Maxillofac Surg. 2020;78(3):440-5.
52. Chitre S. Dental Sealant placement: A Comparison technique. In: 13th International Conference and Exhibition on Dental Medicine; 2016 Aug 8-10; Toronto, Canada.
53. Guzelce E, Bassi F, Karacer O. Restoring congenitally missing mandibular central incisor using lithium disilicate based resin bonded prostheses: a case report. Oral Health Dental Sci. 2018;2(2):1-3.
54. Oliveira TFL, Oliveira LSAF, Santos L, Mascarenhas C, Lopes N, Dantas P. Responsabilidade civil em odontologia - uma visão por profissionais da área jurídica. Odontol Clín Cient. 2013;12(4):261-4.
55. Brasil. Conselho Federal de Medicina. Resolução N° 2.217/2018, 27 de setembro de 2018. Código de ética médica. [Internet]. Brasília: Conselho Federal de Medicina; 2018. Available from: https://portal.cfm.org.br/images/PDF/cem2019.pdf
56. Silva RHA, Musse JO, Melani RFH, Oliveira RN. Responsabilidade civil do cirurgião-dentista: a importância do assistente técnico. Rev Dental Press Ortodon Ortop Facial. 2009;14(6):65-71.
57. Gutmann IE, Dutra RT. Reações adversas associadas ao uso de preenchedores faciais com ácido hialurônico. Rev Elet Bioc Biotec Saúde. 2018;11(20):7-17.
58. Netto AL, Ruiz AM. Responsabilidade Médica. Rev Bras Oftalmol. 2010;69(2):75-6.
59. Lolli LF, Lolli MCGS, Marson FC, Silva CO, Moreira MA, Silva RHA. Responsabilidade criminal do cirurgião-dentista. Acta Jus. 2013;1(1):17-23.
60. Brasil. Presidência da República. Decreto-Lei N° 2.848/1940, 7 de dezembro de 1940. Código Penal- Artigo 129. Casa Civil. Subchefia para assuntos jurídicos. [Internet]. Rio de Janeiro: Presidência da República; 1940. Available from: http://www.planalto.gov.br/ccivil_03/decreto-lei/del2848.htm
1. Universidade de São Paulo, Ribeirão Preto, SP, Brazil
Institution: Universidade de São Paulo, Faculdade de Odontologia de Ribeirão Preto, Departamento de Estomatologia, Saúde Coletiva e Odontologia Legal, Ribeirão Preto, SP, Brazil.
NLM Analysis and/or data interpretation, Conception and design study, Conceptualization, Data Curation, Final manuscript approval, Formal Analysis, Funding Acquisition, Investigation, Methodology, Realization of operations and/ or trials, Resources, Validation, Visualization, Writing - Original Draft Preparation, Writing - Review & Editing.
JGDP Analysis and/or data interpretation, Conception and design study, Conceptualization, Data Curation, Final manuscript approval, Funding Acquisition, Investigation, Methodology, Realization of operations and/or trials, Supervision, Validation.
RHAS Analysis and/or data interpretation, Project Administration, Supervision, Visualization, Writing - Review & Editing.
Corresponding author: Ricardo Henrique Alves da Silva Avenida do Café, s/n, Bairro Monte Alegre, Ribeirão Preto, SP, Brazil, Zip Code: 14040-904, E-mail: ricardohenrique@usp.br
Article received: May 05, 2021.
Article accepted: July 14, 2021.
Conflicts of interest: none.








 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter