

Original Article - Year 2022 - Volume 37 -
Modified double space technique for augmentation mastopexy
Técnica de duplo espaço modificada para mastopexia de aumento
ABSTRACT
Introduction: Mastopexy with prosthesis remains a challenge for all plastic surgeons. Consistent results are still difficult to obtain for many reasons, and some authors advocate caution due to complications.
Methods: This retrospective study includes 92 consecutive single-stage augmentation mastopexy cases performed by the same surgeon between March 2012 and October 2019.
Results: The median follow-up was 14 months for the augmentation mastopexy group. Three patients (3.3%) had grade III capsular contracture. The revision rate was 25.3%: 7.6% with ptosis recurrence, scar revision in 6.5%, and 1.1% asymmetries. Three patients (3.3%) had ruptured muscle loop fixation, four patients (4.4%) had dynamic deformities.
Conclusion: The modified double-space technique in our experience shows consistent long-term results, and revision/complication rates were similar to some studies but higher than others. Prior breast surgery, smoking, breastfeeding, and previous bariatric surgery do not increase rates of complications and revisions. It can be one of the options for covering and supporting implants in augmentation mastopexy procedures.
Keywords: Mammoplasty; Breast implant; Risk-factors; Postoperative complications; Surgical flaps; Comparative study.
RESUMO
Introdução: A mastopexia com prótese continua sendo um desafio para todos os cirurgiões plásticos. Resultados consistentes ainda são difíceis de se obter por muitas razões e alguns autores defendem a cautela devido a complicações.
Métodos: Este estudo retrospectivo inclui 92 casos consecutivos mastopexia de aumento em tempo único realizados pelo mesmo cirurgião, entre março de 2012 e outubro de 2019.
Resultados: O seguimento médio foi de 14 meses para o grupo de mastopexia de aumento. Três pacientes (3,3%) tiveram contratura capsular grau III. O índice de revisões foi de 25,3%: 7,6% com recidiva da ptose, revisão de cicatrizes em 6,5%, 1,1% de assimetrias. Três pacientes (3,3%) apresentaram ruptura da fixação da alça muscular, quatro pacientes (4,4%) tiveram deformidades dinâmicas.
Conclusão: A técnica modificada de duplo espaço apresenta em nossa experiência resultados consistentes a longo prazo, e as taxas de revisão/complicação foram semelhantes a alguns estudos, mas maiores do que outros. Cirurgia prévia da mama, tabagismo, amamentação e cirurgia bariátrica anterior não aumentam as taxas de complicações e revisões. Pode ser uma das opções para a cobertura e suporte dos implantes em procedimentos de mastopexia de aumento.
Palavras-chave: Mamoplastia; Implante mamário; Fatores de risco; Complicações pós-operatórias; Retalhos cirúrgicos; Estudo comparativo
INTRODUCTION
Since its first description, mastopexy with prosthesis continues to be a challenge for all plastic surgeons since its first description1,2. Consistent long-term results are still difficult to obtain for many reasons, and some authors advocate caution due to complications3,4.
One of the most common reasons for surgical revision is ptosis recurrence, which can have many underlying factors, but the lack of reliable long-term support is the main cause5. The pectoralis major muscle has been studied and used in many techniques with different results6-15, although pure dual-plane is still preferred in most studies16-21. Daniel developed a technique in 2005 that he called “double space,” in which he divides the pectoralis major muscle inferolateral to create a pocket where the implant will be placed8.
The lead author of this study has been using this technique since 2012 and describes his experience in 92 consecutive cases (184 breasts) with two modifications for better pocket stability and consistency of results15,19,22,23.
OBJECTIVES
This study aims to describe a single surgeon’s experience in a modified approach to augmentation mastopexy.
METHODS
This study was conducted following the 1964 Declaration of Helsinki guidelines and later amendments or comparable ethical standards. The patients came from the surgeon’s private clinic who authored the study.
This retrospective study included 92 consecutive cases (184 breasts) of single-stage augmentation mastopexy performed at Hospital Pietá, in Curitiba, PR, by the same surgeon using a modified double space technique between March 2012 and October 2019
Weight and body mass index (BMI) were checked before surgery; smokers were encouraged to quit 30 days earlier. Those who did not comply with the request were denied surgery. Preoperative and postoperative photographs were taken.
All patients received perioperative antibiotics and the application of intermittent lower limb compression devices. Epidural anesthesia associated with sedation was used. No drain was placed in any case. Each patient was discharged on the day of surgery (except when associated surgeries were performed) and encouraged to ambulate soon after sedation wore off. Analgesics and anti-inflammatories were maintained for an average of one week. The surgical bra was indicated for 2 months.
Patients were followed up at 1 day, 1-2 weeks, 1-2-3-6 months, and annually.
Complications were defined as “tissue-related” and “implant-related.” Revisions were defined as minor (performed under local or local anesthesia/sedation for less than 30 minutes) and major (lasting longer than 30 minutes under epidural/sedation). No patient satisfaction assessment was made.
Statistical analysis of the data was performed using chi-square (χ2) analysis and Spearman’s correlation test. Spearman’s correlation test compared revision rates and possible risk factors (smoking, BMI, previous breast surgery, bariatric surgery, and breastfeeding). Statistical significance was defined as p< .05.
Inclusion criteria
Pseudoptosis, grade I or higher breast ptosis (Regnault classification) and hypoplasia.
Exclusion criteria
Significant asymmetry requiring augmentation only on one side and mastopexy on the other. Active smokers.
Surgical description of the double space technique
Patients underwent mastopexy with an inverted or vertical T scar. The implant was accessed through the vertical incision until the pectoralis major fascia was found, then a subfascial pocket was created at the superior and medial pole according to the size of the implant, and breast tissue reduction was performed when necessary.
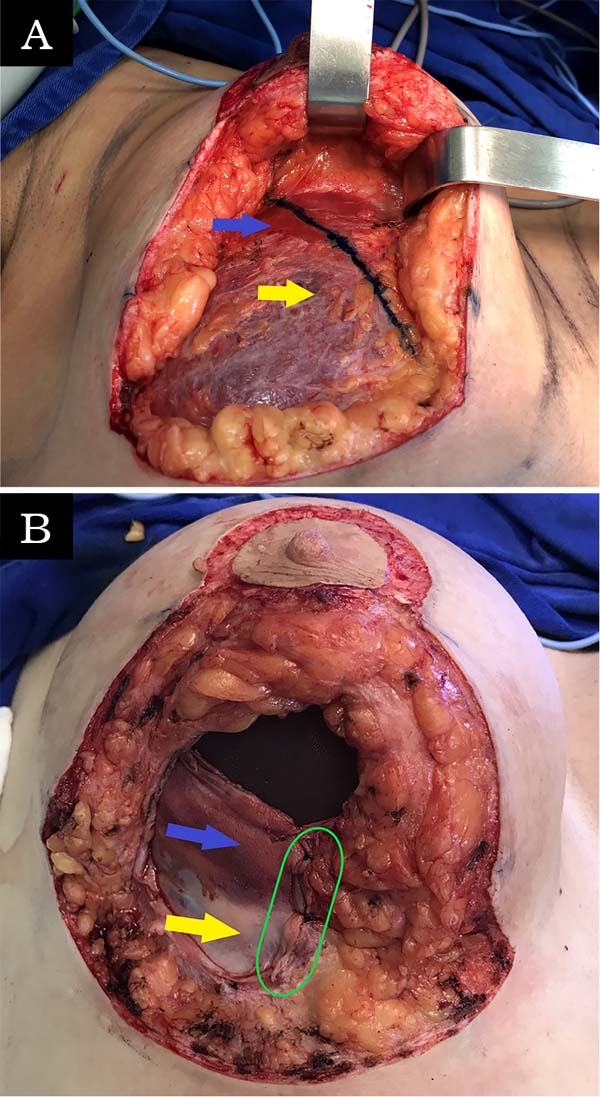
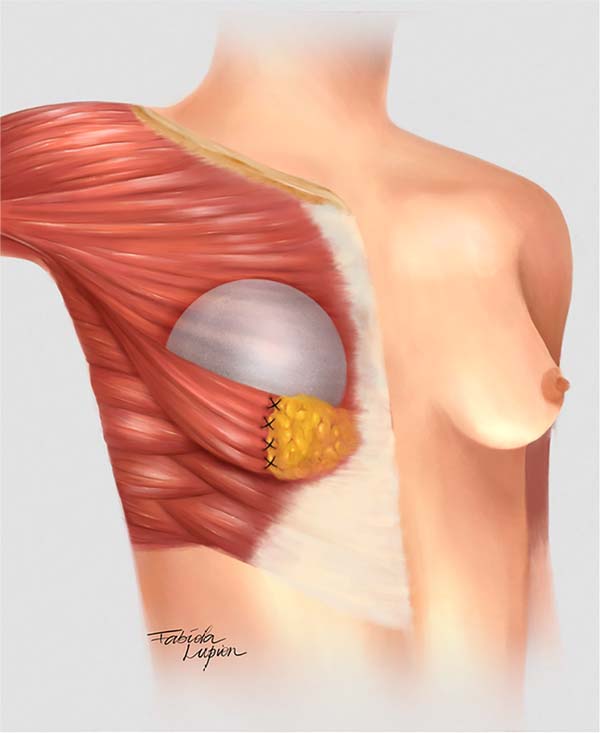
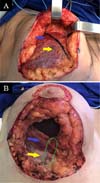
The double space technique was the same as in the cases described by Daniel in 2005. The pectoralis major muscle was divided along the same axis of its fibers 3 cm above its lower border (on average) to the axillary fibers and connected to the adjacent breast tissue. The sternum inserts were sectioned to create an inferolateral submuscular pocket where the implant would be inserted. The size of the pocket varied according to the size of the implant, intending to cover at least 30-40% of the implant, which also facilitated the fixation of the muscle flap to the medial pillar of the breast (Figure 1).
One of the modifications made by the author was the use of a subfascial plane in the superomedial pole, while Daniel used a subglandular approach23.
Muscle flap fixation
The other modification was the fixation of the muscle flap to the medial pillar of the breast tissue to stabilize the pocket using nylon 2.0 in separate “U” sutures (at least 4). An extra locking suture was performed last year to reinforce the suture line and prevent muscle tearing.
Mastopexy
The implant plane was subfascial at the superomedial pole and submuscular at the inferolateral pole. Then, approximation of the breast pillars using 2.0 nylon so that the implant was covered by two layers: muscle flap and mastopexy breast parenchyma. Subcutaneous cellular tissue and skin were closed in layers with monocryl 3.0 and 4.0, surgical glue (Dermabond®) was used. The nipple was exposed with the open technique. The drain was not used in any case.
Secondary cases
The muscle flap had to be made differently in patients with previous breast augmentation surgery. If the anterior plane was subglandular or subfascial, the muscle flap was created and left separate from the glandular tissue. If the anterior plane was submuscular, most patients had significant retraction of the pectoralis major muscle, so the flap was also created with a portion of the inferolateral capsule (Figure 2).
RESULTS
The age of the patients ranged from 17 to 54 years, with a mean of 41.67 years. The mean BMI was 24.2. The implant size ranged from 150 ml to 450 ml (average 281 ml), all high-profile or ultra-high profile textured rounds. The mean follow-up was 14 months (Table 1).
| Augmentation mastopexy (92 patients) | |
|---|---|
| Age (years) | 17-54 (41.61) |
| Follow-up (months) | 6-72 (14) |
| BMI | 24.22 (média) |
| Smokers | 6 (6.52%) |
| Previous breast surgery | 39 (42.39%) |
| Post-bariatric | 5 (5.44%) |
| Breast-feeding | 49 (53.26%) |
BMI: Body Mass Index
Five patients (5.4%) were post-bariatric, and 49 patients (53.3%) had a history of breastfeeding. Only six patients (6.5%) were smokers, and 39 patients (42.4%) had previous breast surgery (augmentation, augmentation, mastopexy or reduction mammoplasty).
All patients underwent a double space approach for internal implant support, and most cases were performed with inverted T mastopexy (90.25%) and some (9.75%) with only a vertical and periareolar scar.
The complication rate was 27.17%. There were no cases of postoperative infection or necrosis of flaps or nipple-areolar complex (NAC). Only one patient (1.09%) had NAC suffering in a secondary mastopexy, which was completely resolved with cilostazol 100 mg/day for 10 days associated with three hyperbaric chamber sessions.
There were more tissue-related complications than implant-related complications (Table 2). Three patients (3.26%) had grade III capsular contracture: one underwent capsulectomy with replacement of implants, the second underwent explantation with capsulectomy and new mastopexy, the third was treated only with Singulair 10 mg/day for 3 months, with improvement to a grade I contracture and no reoperation was necessary.
| Total | % | Conduct | |
|---|---|---|---|
| Implant | |||
| Capsular contracture | 3 | 3.26 | Capsulectomy and implant replacement/Explant/Singulair 10 mg/day |
| Asymmetry | 1 | 1.09 | Lipoinjection/scar revision |
| Tissue | |||
| Recurrent ptosis | 7 | 7.61 | New mastopexy |
| Unsightly scars | 6 | 6.52 | Scar review |
| Dynamic deformity | 4 | 4.35 | Lipoinjection |
| Loss of muscle strap attachment | 3 | 3.26 | New fixation with locking suture |
| NAC suffering | 1 | 1.09 | Hyperbaric chamber and cilostazol 100 mg/day |
NAC: Nipple-areolar Complex
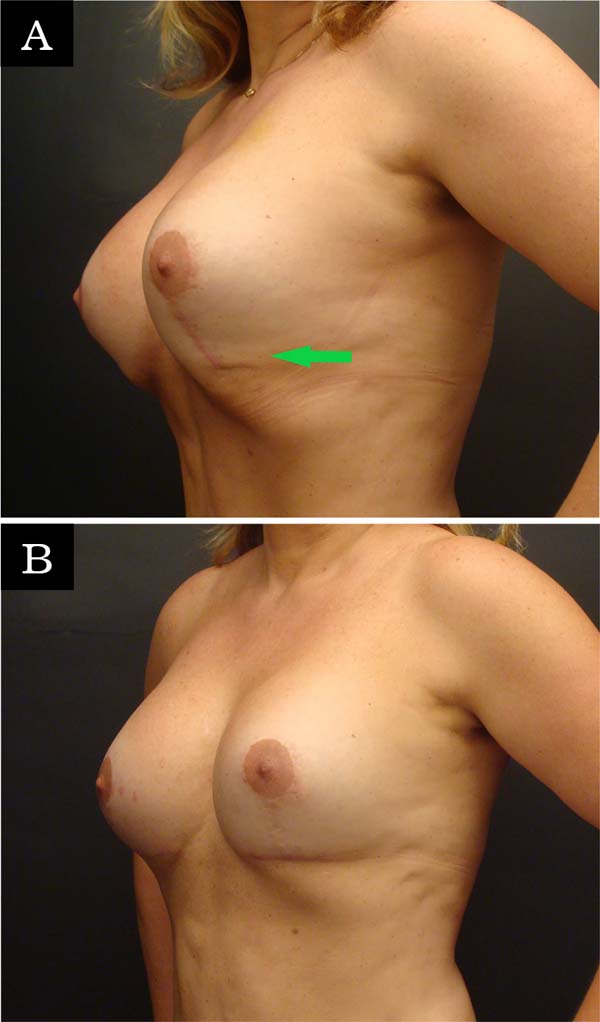
The most common complication was recurrent ptosis (7.61%), and all patients underwent a new mastopexy for correction. Unaesthetic scars were the second most common complication (6.52%), and some patients were reviewed with only local anesthesia, but the epidural was used when another complication was treated in the same patient. It was found in three patients (3.26%) the rupture of fixation of the muscle flap and loss of support for this reason; all were surgically revised for new fixation with locking suture. In four patients (4.35%), lipoinjection was necessary for the inferolateral quadrant due to the dynamic deformity of the muscular loop (Figure 3).
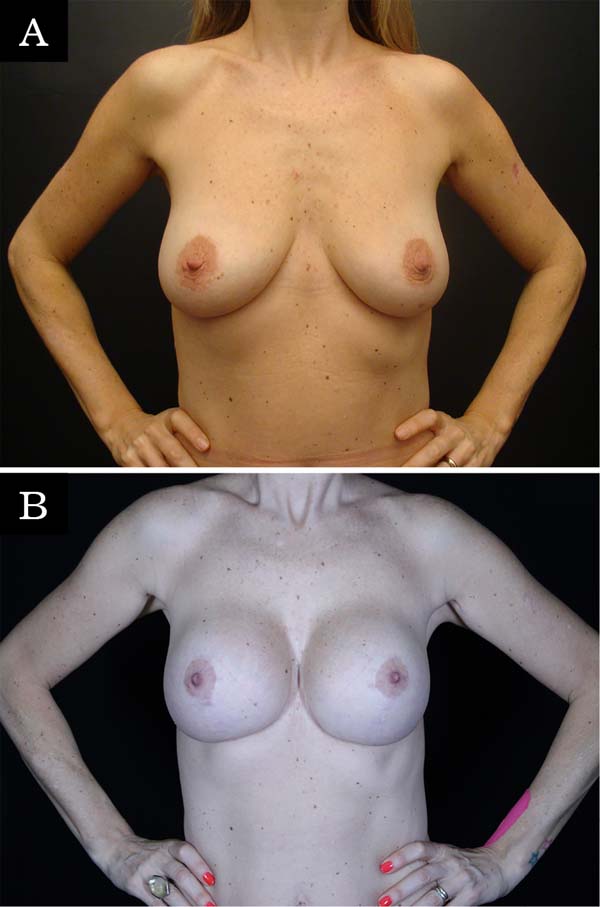
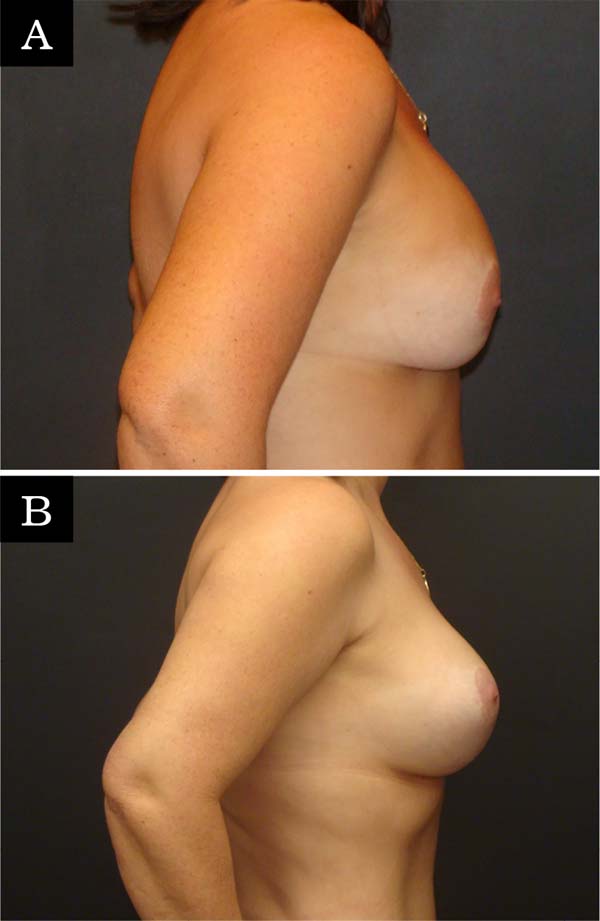
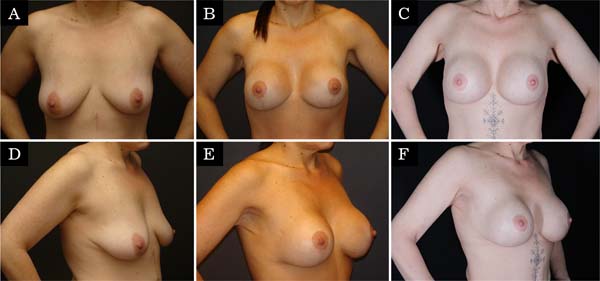
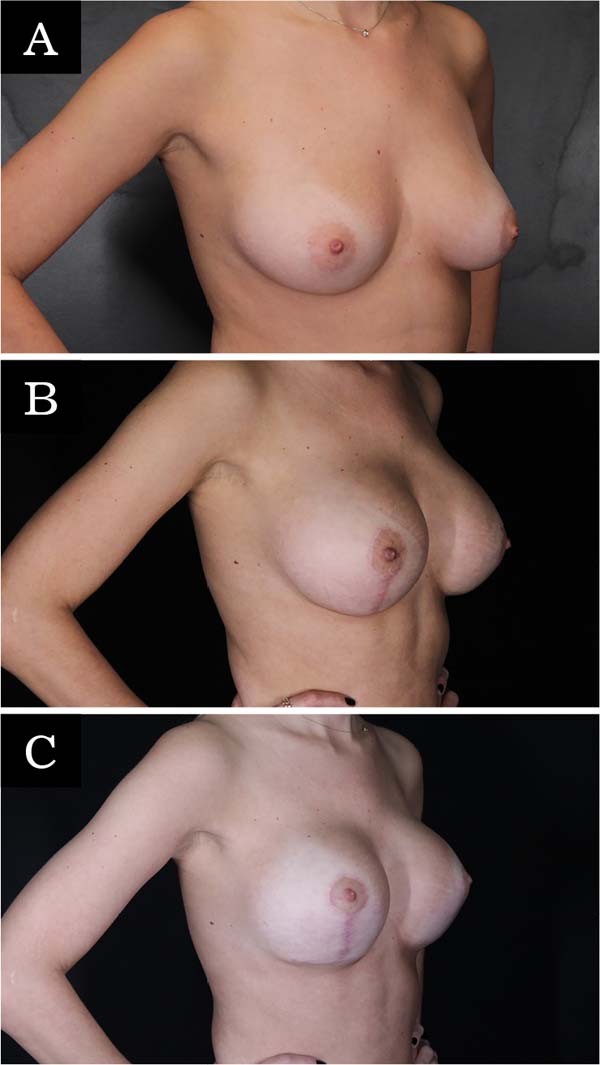
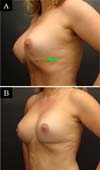
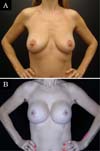
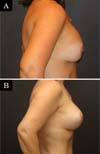
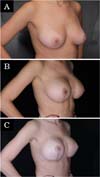
The revision rate was 25.3% (Table 3), and two (8.7%) of these were considered minor because they were performed under sedation/local or local only lasted less than 30 minutes. Revisions performed under sedation/epidural were considered major, took more than 30 minutes, and were performed in deeper structures (breast tissue, muscle). Four patients (4.4%) had two associated complications (Table 3). Long-term results are shown in primary (Figure 4), secondary (Figure 5) and post-bariatric (Figure 6) patients.
| Augmentation mastopexy (92 patients) | % | Revision | |
|---|---|---|---|
| Recurrent ptosis | 7 | 7.61 | 7 |
| Capsular contracture | 3 | 3.26 | 2 |
| Unsightly scars | 6 | 6.52 | 6 |
| Dynamic deformity | 4 | 4.35 | 4 |
| Asymmetry | 1 | 1.09 | 1 |
| Loss of muscle strap attachment | 3 | 3.26 | 3 |
| NAC suffering | 1 | 1.09 | 0 |
| Size (very large) | 0 | 0.00 | 0 |
NAC = nipple-areolar complex
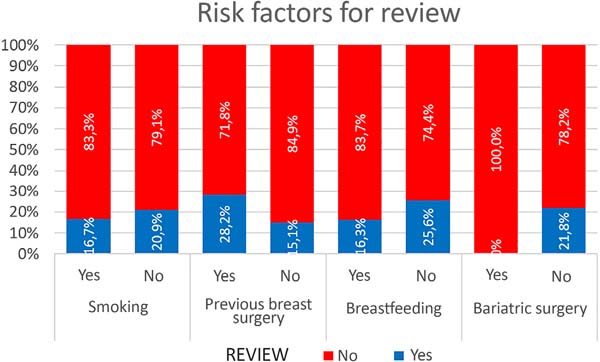
We found no statistically significant difference in revision rates in risk factors such as smoking, previous breast surgery, post-bariatric patients and breastfeeding history (Figure 7).
DISCUSSION
Daniel first described the double space technique in 2005 in a series of 320 patients in which he used it for primary and secondary cases for breast augmentation and augmentation mastopexy. Daniel claimed that his technique combined the advantages of the submuscular plane (better coverage at the lower pole, more support, less rippling) and subglandular (faster recovery, no dynamic deformities, no implant displacement or waterfall deformity). Furthermore, according to him, the disadvantages of each were eliminated8.
One of the problems in breast surgery studies is the variety of surgical techniques and maneuvers to treat muscle, breast tissue and skin, not to mention the implant itself (size, profile, filling, texturing)5,16,24-26. Therefore, these factors can influence the result and the revision/complication rates17,27,28.
There is also the patient perspective, of course, which can increase the demand for surgical revisions even when the result is more than satisfactory in the eyes of the surgeon. Another aspect to consider is the cultural differences between countries, when accepting certain results may be lower. In Brazil, women are much more demanding, especially regarding filling the upper pole and firmness of the breasts, even with a previous history of great weight loss and breastfeeding. Therefore, requests for revisions to achieve these goals are quite common in our environment17,25,26 (Figure 8).
One of the only systematic reviews evaluating single-step augmentation mastopexy found significant inter-study heterogeneity in complications and reviews25. The most common individual complication was recurrent ptosis, with a pooled rate of 5.2%, as in other studies9,18,20. In the present study (7.61%), it was also the most common complication. Therefore, all efforts to avoid this complication should be made. Many authors have published different forms of implant support11-13,15,26,29,30.
In 2018, an author made a similar approach, but with a different muscle flap fixation and only in secondary cases, and did not mention the work of Daniel (2005), although the muscle flap was the same15. The fixation used in the current study is with at least 4 stitches on the medial pillar with a locking suture to prevent the muscle flap from being torn by the suture line. More sutures can help prevent muscle tears found in three patients, even with 4 or more sutures. In the last year, a blocking suture was added in an attempt to solve this problem, and so far, this complication has not been observed anymore.
Munhoz (2019) published a study with a very similar technique called CRIMS (composite reverse inferior muscle sling), but there was no report of dynamic deformity or recurrent ptosis in this study. This is probably due to a small sample (32 patients), in which only primary cases were operated. The size of the pocket that this author created for implant insertion varied according to the size of the implant, covering at least 50-60% of the implant. A similar approach was used regarding pocket size, but as the mean size was larger (281 ml compared to 255 ml), the desired coverage was 30-40% of the implant11.
Daniel claimed that there was no dynamic deformity with this technique, but the author found it in 4.35% of the patients (4 cases) in the lower pole, and these patients had to undergo lipoinjection for correction. It is possible that this complication is related to incomplete detachment of the pocket and also to the position of the pectoralis major muscle and its relationship with the inframammary fold (IMF), as claimed by Maclin et al. (2015)31. The authors state that the lateral extension of the IMF can be difficult to identify and varies according to the size of the breast and the size of the patient.
On average, the IMF is 2 cm lower than the lower limit of the pectoralis major muscle, and the lower muscular origin can be found in the 5th, 6th or 7th rib32. Sometimes, especially in larger implants, the detachment must include serratus muscle. It was also observed in this series that, on the left side, the insertion of the pectoralis major muscle is generally more cranial than on the right side. Since the technique presented in this study is based on the support of a pectoralis major flap inferolateral, the muscle fibers are not divided as in the dual plane technique. This is the main reason why authors using dual planes would not have this complication in the inferolateral pole because muscle contraction acts differently from the current technique3,5,16,17,20,21.
No cases of waterfall deformity (Waterfall/Snoopy nose) were found when the implant is in the right position and the breast parenchyma “falls” or slides over it15,22. This appears more in a long-term evaluation, irrespective of implant texture and insertion plane. The technique creates two different implant contact surfaces, a subfascial (superomedial half) and a submuscular (inferolateral half), which could reduce this complication’s chances. In addition, the modification applied by the author can help avoid this deformity since there is a fixation between muscle and breast tissue. Other studies may consider waterfall deformity as pseudoptosis or recurrent ptosis, but they are different entities4.
There was no skin flap or NAC necrosis in this case series, only one case of NAC suffering resolved with hyperbaric chamber sessions and cilostazol. Although almost 40% of the patients had previous breast surgeries, these patients are notoriously more likely to have blood flow compromised with tissue atrophy4. This demonstrates the safety of the technique concerning the blood supply of the flaps.
Scarring is one of the most common complications of any mammoplasty, whether reduction or mastopexy. When breast augmentation tension is applied to the same procedure as augmentation mastopexy, small dehiscence and enlarged scars are more likely to occur. This was the second most common cause of complication (6.52%). Some revisions were minor and performed under local anesthesia (small areas), but others required an epidural, especially when associated with longer scarring or other problems (recurrent ptosis, dynamic deformities, or other areas). Other authors also found this, and some had higher rates, probably due to the use of implants larger than the average of this series (281 ml)6,9,10,11,14-17,20,21,29,33,34.
Recurrent ptosis is the reason why there are so many different techniques for sagging breasts. When augmentation is added to mastopexy, extra elements are added to the equation: implant size and texture, position (under or above the muscle), the interconnection between the implant and breast tissue (waterfall deformity), amount of residual breast tissue. The mastopexy technique is also important for long-term results. What is the real cause of recurrent ptosis? Implant, mastopexy or both25?
The rate of recurrent ptosis found was similar to some studies9,15,29,30,34, but significantly higher than others10,17,18,20,21,33,35,36. Again, other studies with similar approaches had lower rates of recurrent ptosis, probably because they were small series of patients, short follow-up or only primary cases11,15. The muscle flap remains active even after many years of the postoperative period, demonstrating that the technique provides adequate support without relying solely on mastopexy.
Although 42.4% of the cases in this series had previous breast surgeries, 5.44% were post-bariatric and more than half (53.26%) of patients had a history of breastfeeding; there was no statistically significant difference between these variables in the rate of complications/revisions. The rate of smokers (6.52%) was small and did not influence this rate of complications and revisions. Most studies do not assess these factors as determinants of outcomes, complications, and reviews. Calobrace et al. (2013)17, on the other hand, evaluated this variable and found a higher complication rate in secondary than in primary cases.
The complication rate was 27.5%, similar to some studies16,17,20,21,29,35, but higher than others11,15,18. These last studies were of a small series of cases (around 30) or had a short follow-up13, which could explain the lower rate of complications. Two studies had very high complication rates (about 50%)9,36, perhaps because both authors considered minor changes as complications: minor hematomas, superficial skin vesicles, minor dehiscence, discrete skin remnants (ears) even areolar overgrowth. Most studies did not consider these factors as complications.
The overall review rate was 25.5%, similar to that of Calobrace et al. (2013)17 and Hubbard (2019)37, but higher than that of other authors15,18,33,35. This finding has many nuances: short follow-up, small series, and only primary cases are just some of them. Cultural differences in the acceptance of results can also be brought to light, which means that some patients go for a review with very good results already effectively26. For example, Spear et al.18 had 54% of patients who completed a satisfaction survey saying that they would want revision surgery, but their revision rate was only 14%.
Calobrace et al.17 found approximately 30% of revision surgery in secondary cases, 10% more than in primary cases. As almost 40% of the surgeries in this series were secondary, a higher revision rate would be expected considering the difficulty of these cases (change of planes, poorly placed scars, devascularized tissues, parenchymal atrophy and capsular contracture4. However, no statistical difference was found significant in secondary cases.
Study limitations
It is a retrospective study; no satisfaction rating was made. The technique has evolved over the years to make the pocket and fix the muscle flap to improve results. The author first performed the double-space technique in 2012; these patients include their learning curve. There are no objective measures to assess pre/post-operative or short/long-term outcomes.
This study tolerates relatively short follow-up times to maximize the enrollment rate.
CONCLUSION
The modified double-space technique in our experience shows consistent long-term results, and revision/complication rates were similar to some studies but higher than others. Prior breast surgery, smoking, breastfeeding, and previous bariatric surgery do not increase rates of complications and revisions.
It can be one of the options for covering and supporting implants in augmentation mastopexy procedures.
REFERENCES
1. Gonzalez-Ulloa M. Correction of hypotrophy of the breast by exogenous material. Plast Reconstr Surg Transplant Bull. 1960;25:15-26.
2. Regnault P. The hypoplastic and ptotic breast: a combined operation with prosthetic augmentation. Plast Reconstr Surg. 1966;37(1):31-7.
3. Spear S. Augmentation/Mastopexy: “Surgeon Beware”. Plast Reconstr Surg. 2003;112(3):905-6.
4. Handel N. Secondary mastopexy on the augmented patient: a recipe for disaster. Plast Reconstr Surg. 2006;118(7 Suppl):152S-63S.
5. Spear SL, Dayan JH, Clemens MW. Augmentation mastopexy. Clin Plast Surg. 2009;36(1):105-15.
6. Auersvald A, Auersvald LA. Mastopexy and breast augmentation using a pectoral muscle loop. Aesthetic Plast Surg. 2011;35(3):333-40.
7. Borovlkov A. Use of myofascial flaps in aesthetic breast surgery. Aesthet Surg J. 2004;24(4):331-41.
8. Daniel MJB. Inclusão de Prótese de Mama em Duplo Espaço - Prêmio Georges Arié 2004. Rev Bras Cir Plást. 2005;20(2):82-7.
9. Karacaoglu E. Single stage augmentation mastopexy: a novel technique using autologous dermal graft. Ann Plast Surg. 2009;63(6):600-4.
10. Khan UD. Augmentation mastopexy in muscle-splitting biplane: an outcome of first 44 consecutive cases of mastopexies in a new pocket. Aesthetic Plast Surg. 2010;34(3):313-21.
11. Munhoz AM, Marques Neto A, Ferrari O. Single-Stage Augmentation Mastopexy With Composite Reverse Inferior Muscle Sling Technique for Autologous Reinforcement of the Inferior Pole: Technical Refinements and Outcomes. Aesthet Surg J. 2020;40(6):NP356-73.
12. Ono MT, Kerner BM. Four-step augmentation mastopexy: Lift and augmentation at single time (LAST). Plast Reconstr Surg Glob Open. 2019;7(11):e2523.
13. Procópio LD, Silva DDP, Rosique R. Implante submuscular em duplo bolso para mastopexias de aumento. Rev Bras Cir Plást. 2019;34(2):187-95.
14. Steinbacher DM, Singh N, Katz R, Khalifeh M. Augmentation- mastopexy using an autologous parenchymal sling. Aesthetic Plast Surg. 2010;34(5):664-71.
15. Valente DS. Reverse-Muscle Sling Reduces Complications in Revisional Mastopexy-Augmentation. Aesthetic Plast Surg. 2018;42(5):1202-12.
16. Beale EW, Ramanadham S, Harrison B, Rasko Y, Armijo B, Rohrich RJ. Achieving predictability in augmentation mastopexy. Plast Reconstr Surg. 2014;133(3):284e-92e.
17. Calobrace MB, Herdt DR, Cothron KJ. Simultaneous augmentation/mastopexy: a retrospective 5-year review of 332 consecutive cases. Plast Reconstr Surg. 2013;131(1):145-56.
18. Spear SL, Pelletiere CV, Menon N. One-stage augmentation combined with mastopexy: aesthetic results and patient satisfaction. Aesthetic Plast Surg. 2004;28(5):259-67.
19. Spring MA, Hartmann EC, Stevens WG. Strategies and Challenges in Simultaneous Augmentation Mastopexy. Clin Plast Surg. 2015;42(4):505-18.
20. Stevens WG, Stoker DA, Freeman ME, Quardt SM, Hirsch EM, Cohen R. Is one-stage breast augmentation with mastopexy safe and effective? A review of 186 primary cases. Aesthet Surg J. 2006;26(6):674-81.
21. Stevens WG, Macias LH, Spring M, Stoker DA, Chacón CO, Eberlin SA. One-Stage Augmentation Mastopexy: A Review of 1192 Simultaneous Breast Augmentation and Mastopexy Procedures in 615 Consecutive Patients. Aesthet Surg J. 2014;34(5):723-32.
22. Frame J. The waterfall effect in breast augmentation. Gland Surg. 2017;6(2):193-202.
23. Graf R, Bernardes A, Rippel R, Araujo LR, Damasio R, Auersvald A. Subfascial breast implant: a new procedure. Plast Reconstr Surg. 2003;111(2):904-8.
24. Karnes J, Morrison W, Salisbury M, Schaeferle M, Beckham P, Ersek RA. Simultaneous breast augmentation and lift. Aesthetic Plast Surg. 2000;24(2):148-54.
25. Kavanin N, Jordan SW, Rambachan A, Kim JYS. A systematic review of single-stage augmentation-mastopexy. Plast Reconstr Surg. 2014;134(5):922-31.
26. Sarosiek K, Maxwell PG, Unger JG. Getting the Most Out of Augmentation-Mastopexy. Plast Reconstr Surg. 2018;142(5):742e-59e.
27. Spear SL, Low M, Ducic I. Revision augmentation mastopexy: indications, operations, and outcomes. Ann Plast Surg. 2003;51(6):540-6.
28. Qureshi AA, Myckatyn TM, Tenenbaum MM. Mastopexy and mastopexy- augmentation. Aesthet Surg J. 2018;38(4):374-84.
29. Ismail KT, Ismail MT, Ismail TA, Ismail AT. Toth BA. Triple-Plane Augmentation Mastopexy. Plast Reconstr Surg Glob Open. 2019;7(8):e2344.
30. Migliori F. ‘‘Upside-down’’ augmentation mastopexy. Aesthetic Plast Surg. 2011;35(4):593-600.
31. Maclin II MM, Deigni OA, Bengtson BP. The Laminated Nature of the Pectoralis Major Muscle and the Redefinition of the Inframammary Fold: Clinical Implications in Aesthetic and Reconstructive Breast Surgery. Clin Plast Surg. 2015;42(4):465-79.
32. Madsen RJ Jr, Chim J. Variance in the origin of the pectoralis major muscle: implications for the implant-based breast reconstruction. Ann Plast Surg. 2015;74(1):111-3.
33. Doshier LJ, Eagan SL, Shock LA, Henry SL, Colbert SH, Puckett CL. The Subtleties of Success in Simultaneous Augmentation-Mastopexy. Plast Reconstr Surg. 2016;138(3):585-92.
34. Camargo VJ. Mamoplastia secundária com ressecção em monobloco e neoposicionamento do implante no espaço retropeitoral parcial. Rev Bras Cir Plást. 2019;34(3):315-23.
35. Forcada EM, Fernandez MC, Aso JV, Iglesias IP. Augmentation mastopexy: Maximal reduction and stable implant coverage using four flaps. Aesth Plast Surg. 2014;38(4):711-7.
36. Tessone A, Millet E, Weissman O, Stavrou D, Nardini G, Liran A, Winkler E. Evading a surgical pitfall: mastopexy--augmentation made simple. Aesthet Plast Surg. 2011;35(6):1073-8.
37. Hubbard TJ. Vertical Augmentation Mastopexy with Implant Isolation and Tension Management. Plast Reconstr Surg Glob Open. 2019;7(6):e2226.
1. Private practice, Curitiba, PR, Brazil.
| COLLABORATIONS | |
|---|---|
| LRRA | Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Investigation, Methodology, Project Administration, Realization of operations and/or trials, Supervision, Writing - Original Draft Preparation, Writing - Review & Editing. |
| WMI | Analysis and/or data interpretation, Data Curation, Final manuscript approval, Methodology, Visualization, Writing - Review & Editing. |
Corresponding author: Luiz Roberto Reis Araujo, Alameda Presidente Taunay, 1820, Merces, Curitiba, PR, Brazil, Zip Code 80430-042, E-mail: drluiz@drluizaraujo.com.br
Article received: March 16, 2021.
Article accepted: December 13, 2021.
Conflicts of interest: none.









 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter