

Articles - Year 1997 - Volume 12 -
Head and Neck Reconstructions with Prolene Mesh
Tela de Prolene - Utilização em Reconstruções de Cabeça e Pescoço
ABSTRACT
Independently of their composition, alloplastic materials when introduced into the organism cause an acute inflammatory reaction Jollowed by a chronic process. The authors studied the introduction of a strip of prolene mesh in "U" type rats and then by light microscopy accompanied the reactions which occurred an days 5,10,14,24,30 and 40, so that observation was possiblefrom the acute inflammatory process until the imp/ants's integration. They report the satisJactory outcome of the use of the mesh in 3 clinical cases; indicated in 2 for suspension and in 1 for support. Mention is made that the prolene mesh obeys all the criteria laid down by ScaleJor the ideal implant, except for causing an inflammatory reaction, which is acute initially, Jollowed by repair with resolution and integration of the implant. They conclude citing the advantages oJthe mesh: the good results obtained, histological evidence of integration, absence of scars in other sites, and it is a good option for suspension of ptosed structures, as well as for tissue support.
Keywords: Prolene Mesh, Alloplastic Materials
RESUMO
Os materiais aloplásticos, independentemente de sua constituição, determinam uma resposta inflamatória quando introduzidos no organismo, em princípio, de caráter agudo, seguido de processo crônico. Os autores fazem um estudo em ratos tipo "U" nos quais introduzem uma fita de tela de prolene. Através de microscopia óptica, fazem o acompanhamento de reações que ocorrem no quinto, décimo-quarto, trigésimo e quadragésimo dias, sendo possível a observação desde o processo inflamatório agudo até a fase de integração do implante. A seguir, demonstram a utilização da tela em três casos clínicos, com a qual teve indicação de suspensão em dois casos e sustentação em um, com resultados satisfatórios. Citam que a tela de prolene obedece a todos os critérios de Scale como um implante ideal, com exceção do fato de causar reação inflamatória inicialmente aguda, caminhando para um processo de reparação com características resolutivas de integração do implante. Concluem pelos bons resultados obtidos, pela comprovação da integração em cortes histológicos e por não deixar cicatrizes em outras áreas, tratar-se de uma boa opção para suspensão de estruturas ptosadas, assim como para sustentação tissular.
Palavras-chave: Tela de Prolene, Materiais Aloplásticos
The utilization of alloplastic materials (i.e. silicone prostheses, polypropylene mesh etc.) in plastic surgery has been widely accepted. Whatever the nature ofthe material employed, some inflammatory reaction is bound to occur.
Therefore, there is no such thing as an inert inclusion.
A fact which that we become aware ofthe inflammatory process involved in each case(9).
In situations in which tissue suspension or a gain in tissue consistency is necessary, prolene mesh is employed.
Both the prolene and Marlex meshes are composed of polypropylene. However, the former is knit with 2 stitches making it rigid in all directions, where as the Marlex is a mesh, knit with a single stitch which is rigid in one direction and stretchable in the other(8).
The thesis of the present article is a detailed study of the reactions to implantation of prolene mesh in rats, followed by clinical applications.
Review of the Literature
The use ofdevices for facial suspension are mentioned in chronological order as follows: Blair, 1926-Proposes the use offascia lata strips fixed to the commissure of the lips and to the temporal aponeurosis in a case offacial paralysis. The method was also employed by Gillies (1934) and Freeman who accomplished a 2 stage procedure.
Bunnell, 1937 and McLaughlin, 1952 - Utilized the fascia lata with minor modifications to suspend the face.
McLaughlin fixed one ofthe extremities ofthe fascia to the coroinoid process.
Ragnell, 1958 - Used the extensor tendon ofthe foot to unite the aponeurotic loop surrounding the lip to the fragment of the coronoid process.
AsWey - Associated a masseter muscle flap to McLaughlin's technique.
Trevisani & colleagues, 1982 - Suspended the ear with a strip ofMarlex mesh, in a patient with neurofibromatosis.
Strelzow & colleagues, 1982 - Case reports of facial paralysis reconstructed with polypropylene mesh with good results in 3 patients.
Material and Methods
Ten "U" type rats were utilized for the study. A 3x I cm strip ofprolene mesh was introduced into the animals' backs (Figs. 1,2,3) to be removed on days 5, 10, 17,24,30 and 40 (Fig. 4).
Fig. 1 - 3 x 1cm strips of prolene mesh employed in the study.
Fig.2-Marking of the incision with methylene blue for insertion of the mesh.
Fig. 3 - Insertion of the mesh into the animal'back between the panniculus.
Fig4.- Tissue specimen, including skin, paniculus, mesh and muscles removed from the rat's back.
The resected tissue (skin, panniculus carnosus, mesh and muscles) were fixed in 10% buffered formalin and the paraffin block sectioned (5u) and stained with hematoxylin-eosin (HE).
The tissue reactions were studied by light microscopy so as to obtain standards for the clinical use of prolene mesh.
Microscopic Study
5th Day - An acute inflammatory reaction characterized by edema and capillary dilatation was observed in the dermis; capillary dilatation, swollen endothelium, both mono - and polymorphonuclear infiltration, and deposits offibrin in the panniculus carnosus; edema and mixed infiltrate in the muscles.
Whereas, in the surface in contact with the mesh, the connective tissue was loose, infiltrated with red blood cells, lymphocytes, plasmocytes, neutrophyls, edema, fibrin and numerous polymorphonuclear cells (Fig. 5).
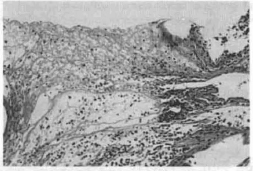
Fig. 5 - Notice Ihe presense of fibrin, permeated by inflammatory infiltrate (both mono and polymorphonuclear), edema and hemorrhagic infiltration (HE lOx 10). - At the level of the mesh implant.
10th Day - In certain areas, granulation tissue is present in the early stage, while in others, proliferation of collagen fibers is already noted (Figs. 6,7) .

Fig. 6 - Voscular proliferation and congestion lymphoplasmocytis infiltrate hemorrhage and edema. Focal area of foreign body giant cell reaction at the level of implantation of the mesh. (HE 1O x 1O).
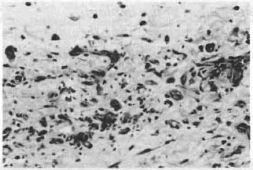
Fig. 7 - Edema and proliferation of fibroblasts at Ihe level of implantation of the mesh (HE 20 x 10).
17th Day - Initial stage of organization of the healing process with the connective tissue being retracted. Edema is decreasing in the dermis and the myxoid connective tissue is proliferating with slight mononuclear infi Itration and vascular neofonnation in the deep tissues (Fig. 8).
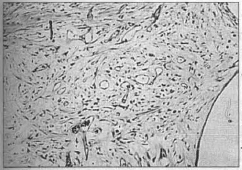
Fig. 8 - Late stage of the inflammatory process, evidence of sligth mononuclear infiltrate besides fibroblastic and vascular proliferation at the level of the implant of the mesh. (HE 10 x 10).
24th Day - Scar with decrease ofedema, contraction and collagen fonnation: slight vascular congestion and deeper down, connective tissue with dilated vessels, varying in diameter with evidence ofanastomoses, appearance of bundles of collagen fibers in various direction (Fig. 9).
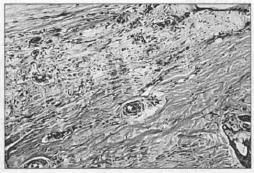
Fig.9 - Notice the reduction in edema, the disorganization of die collagen fibers, at the level of implantation of the mesh. (HE 4x 10).
30th Day - Organized scar: loose connective tissue with areas of (denser) collagen throughout. Presence of dilated vessels besides slight mononuclear inflammatory infiltrate (Fig. 10).
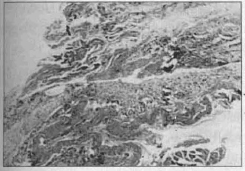
Fig. lO-Late evolutionary stage. There is vascular and fibroblastic proliferation and reduction of edema, at the level of the implanted mesh (HE 4x 10).
40th Day - Organized scar, practically contracted: development of the anterior process (Fig. 11).
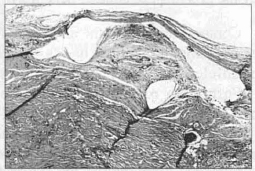
Fig. 11 - Area corresponding to implantation of the mesh with organized scar, at the level of implantation of the mesh (HE lOx 10).
Clinical Cases:
Case 1 - Patient with neurofibromatosis with marked ptosis of the right hemiface (Figs. 12 a,b,c,d).
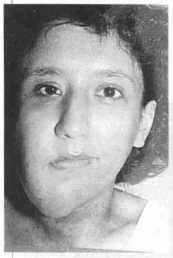
Fig. 12a- Anteriar aspect of neurofibromatosis - facial ptosis.
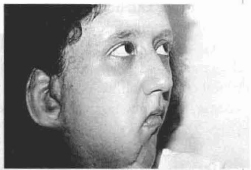
Fig. 12b- Lateral aspect of neurofibromatosis - facial ptosis.
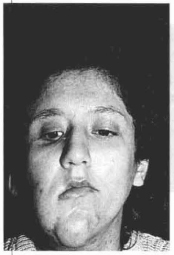
Fig. 12c - Frontal aspect, three months post-implantation of the mesh and reseaction of skin.
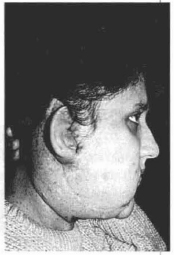
Fig. 12d - Lateral aspect, three months post-implantation.
Case 2 - Paticnt with right facial paralysis complaining of constant drooling and food loss (Figs. 13a,b,c,d,e,f).
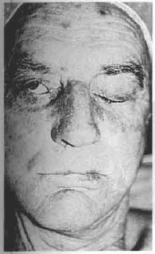
Fig. 13a - Preoperative, anterior aspect. The patient cannot close his right eye.
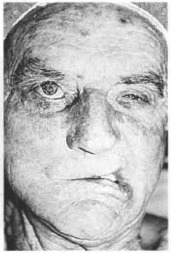
Fig. 13b - Preoperative - The lip is pulled toward the healthy side when contracting the facial muscles.
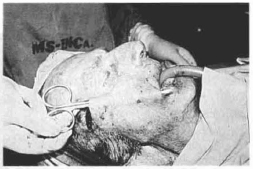
Fig. l3c - At operation, demonstrating the undermining of Ihe skin of the right hemiface for insertion of the mesh, which is anchored to the lip commissure and the temporal fascia.
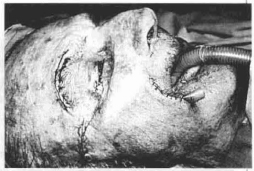
Fig. 13d - Immediate preoperative period. Also notice the suture of the right eyelid where the skin was resected.
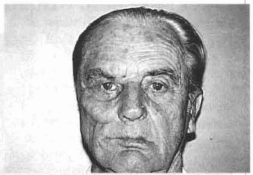
Fig. 13e - 40 days p.o. with the patient at rest. Better positioning of the commissure of the right lip.
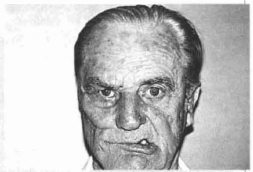
Fig.13f - 40 days p.o., patient contracting the facial muscles. Notice the smaller displacement of the commissure of the right lip in direction to the healthy hemiface.
Case 3 - Patient undergoing resection of a large tumor involving the entire lower lip. Initially, reconstruction was accomplished with a scalp flap. Since the latter was unsuccessful, a deltoid-pectoral flap with prolene mesh was implanted in its extremity for reconstruction of the lower lip (Figs. 14 a-h).
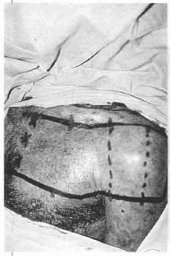
Fig. 14a - Marking of the deltoid-pectoral flap. The dotted line demonstrates the area to be raised and folded over itself with the mesh.

Fig. 14b - Elevation of the distal portion of the subfascial flap. Notice the 2 strips of prolene mesh.
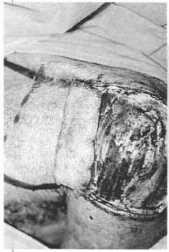
Fig. 14c -Extremity of the flap folded over itself making a "sandwich" with the mesh.
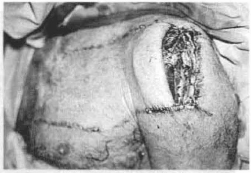
Fig. 14d - Resuture of the extremity of the flap in the donor area itself and autonomization of the remainder.
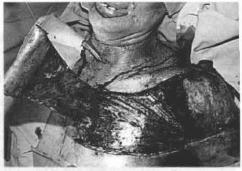
Fig. 14e - Reintervention 15 days after autonomization of the flap with its elevation with the mesh "sandwich" in its extremity.
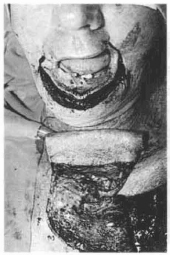
Fig. 14f - Preparation of the receptor area in projection of the lower lip to be reconstrueted. Notice the detail of the flap folded to tailor the lining and covering.
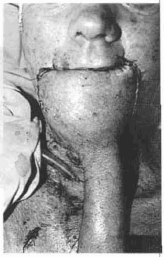
Fig. 14g - Sutlire of the flap in the receptor area tailoring the lower lip.
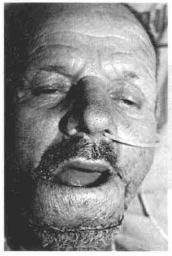
Fig. 14h - Notice the flap forming the lower lip after section of the pedicle.
Discussion
The study in rats demonstrated trat during the first 5 days after insertion of the mesh, an intense acute inflammatory process occurs with the participation of polymorphonuclear cells and fibrin deposits and on the 10th day, when collagen fibers appear, the implant is considered integrated.
On the 24th day, collagen fibers in various directions arc revealed. Cameron & Taylor(3), also observed this with Marlex mesh in which they found circularly disposed, and disorganized collagen fibers. However, these authors mention a better disposition ofthe collagen fibers using carbon mesh.
Starting on day 30, the scar is considered organized.
Strelzow & colleagues(11), mention the possibility of rejection of alii plastic materials. We do not agree with this because what really happens concerning the implants, is an acute inflammatory process followed by a chronic inflammation with resolution. When speaking of rejection, the immunological reaction should be considered, which is defined genetically as the rejection by the organism of another person's or species' tissue(9).
Contamination with the animal's skin occurred in 2 cases promoting a foreigh body giant cell reaction which paralleled the organization process.
Formation of microabscesses occurred in only one case.
Concerning our clinical cases, the prolene mesh has proved to be an interesting material because no major complications occurred and because of the satisfactory results achieved.
According to Scales(9), the ideal conditions for inclusion materials arc defined as follows:
- be chemically inert;
- incapable of modification by organic liquids and tissues;
- incapable of provoking foreign body reaction or inflammation;
- incapable of producing cancer;
- incapable of producing allergic or hypersensitivity reactions;
- capable of resisting mechanical forces;
- capable of being manufactured in any desired shape;
- capable of being sterilized.
The mesh used by us complies with all of Sales criteria, except for the inflammatory process. The latter however, is transitory and determines the integration of the implant in the hosl. A fact confirmed by the good clinical results obtained, together with the microscopic data observed.
In their series of 3 patients with facial palsy, Strelzow & colleagues(11), mention that the static nature of the suspension, diminishes the chance both of exposure of the implant by movement and of provoking the foreign body reaction, so long as the mesh is placed between 2 healthy tissues.
Our indications for placing the mesh were for suspension in a patient with facial palsy and in one with neurofibromatosis. Both reported postoperative improvement in salivary and food continence. In OUI other case, the mesh was placed as a "sandwich" at the extremity of the deltoid-pectoral flap autonomized for reconstruction ofthe lower lip in a patient in whom other types of reconstruction had already failed. In this case, with the mesh, it was possible to tailor a flap with good consistency and in this way, prevent the passive loss of saliva.
Conclusions
Light microscopy demonstrated that with the prolene mesh, the animal organism initially reacts with an acute inflammatory process that progresses to scar organization with total integration.
In our series, no complications with the prolene mesh occurred and satisfactory results were obtained both with suspension as well as with support.
During the period observed, the prolene mesh maintains its function of suspension using a simple operative procedure and with the added advantage of not causing other scars sucb as occur when fascia lata is employed.
ACKNOWLEDGMENTTO
Dr. Washington & Dra. Denise of the Pathology Service of the INCa - RJ
References
1. AREN A.J. et aL Soft tissue response to proplast: quantitation of scar ingrowth. Plast. Reconstr. Surg. Baltimore, v.61 p.214, 1978.
2. BAKER J.L. et al. Occurrence and activity of myofibroblast implants. Plast. RecoDstr. Surg. Baltimore, v.68, p.905.1981.
3. CAMERON A.E., TAYLOR D.E. Carbon-fibre versus MarJex mesh in the repair of experimental abdomimal wall defects in rats. Br. J. Surg. v.72, n.8, aug., p.648· 650, 1985.
4. JOHNSON G. W. Central cone reduction mammoplasties and Marlex suspension of breast tissue. Aesthetic Plast. Surg. New York, v.5, n.l, p.77-84, 1981.
5. KYONO M. et al. Repair of scalp defects using a tissue expander and MarJex mesh. Plast. ReCoDstr. Surg.Baltimore, v.89, n.2, p.349-352, 1992.
6. MARQUES A. Analise critica do comportamento das Inc1usoes: a resposta inOamtoria e reparacional. Rev. Soc.Bras. Cir. Plast. Sao Paulo, v.6, n.l/2. jan.lago., p.5154, 1991.
7. MCCARTHY T. et al. Plastic Surgery. Philadelphia: WB Saunder Company, 1990 T.1.
8. MCCORMACK M.P. Uso de materiais protéticos na reconstrução da parede torácica. Clínicas Cirurgicas da America do Norte. v.5, p.1053-1063, 1989.
9. MELEGA J.M. Cirurgia plastica reparadora e estetica. Rio de Janeiro: Medica e Cientifica, 1988.
10. RUDOLPH R. Complications of surgery for radiotherapy skin damage. Plast. Reconstruct. Surg. Baltimore, v.70, n.2, aug.,p.179-1785,1982.
11. STRELZOW VV et at. Reconstruction of the paralyzed face with the polypropylene template. Arch. Otolaryngol. v.109, n.3, mar., p 140-144, 1983.
12. TREVISANI T.P. et al. Neurofibroma of the ear: function and aesthetics. Plast. Recoostr. Surg. Baltimore, v.70, n.2.aug., p.217-219, 1982.
I - Resident, Division of Plastic Surgery, lnstituto Nacional de Cancer (INCa), Rio de Janeiro-RJ
II - StaffMember, Division of Plastic Surgery, INCa, Rio de Janeiro-RJ
ADDRESS FOR CORRESPONDENCE
Dr. Hemane Sad Medina
Rua Pedro, 1 19/720 - Centro
20060-050 - Rio de Janeiro-RJ


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter