

Case Report - Year 2021 - Volume 36 -
The challenges in the treatment of large burned and polytraumatized patients with arterial thrombosis in the lower limb during the COVID-19 pandemic: a case report
Os desafios no tratamento de paciente grande queimado e politraumatizado com trombose arterial em membro inferior durante a pandemia de COVID-19: relato de caso
ABSTRACT
Introduction: The COVID-19 pandemic spread rapidly worldwide, bringing the need for emergency actions to control the disease at the collective and individual level. Thus, the Division of Plastic Surgery and Burns of the largest hospital complex in Latin America was physically aimed at the infected. In this context, we find the challenges of treating a large burned and polytraumatized patient, who presented arterial thrombosis in the lower limb, a rare complication associated with the thermal burn. All treatment was performed in a hospital not specialized in trauma care.
Case Report: Case Report: Male patient, 18 years old, without comorbidities, with predominantly third-degree burns of 50% of body surface burned and severe neurotrauma. He presented arterial thrombosis in his right leg 24 hours after the burn. The patient underwent limb amputation and serial surgical interventions for debridement and skin grafting. Despite the prevention protocols, the patient was infected by COVID-19 during hospitalization.
Conclusion: This case focuses on a rare complication related to burn injury, which does not yet have diagnostic criteria and defined prophylactic measures. Besides, the COVID-19 pandemic has impacted various health services areas, and it is essential to share knowledge during this pandemic by seeking adaptations to the crisis situation.
Keywords: Burns; Multiple trauma; Thrombosis; Disseminated intravascular coagulation; SARS virus.
RESUMO
Introdução: A pandemia de COVID-19 se espalhou rapidamente pelo mundo trazendo a necessidade de ações emergenciais para o controle da doença em nível coletivo e individual. Assim, a Divisão de Cirurgia Plástica e Queimaduras do maior complexo hospitalar da América Latina foi fisicamente destinada aos infectados. Neste contexto, encontramos os desafios de tratar um paciente grande queimado e politraumatizado, que apresentou trombose arterial em membro inferior, uma rara complicação associada à queimadura térmica. Todo tratamento foi realizado em hospital não especializado ao atendimento de trauma.
Relato de Caso: Paciente masculino, 18 anos, sem comorbidades, com queimaduras predominantemente de 3º grau de 50% de superfície corpórea queimada e neurotrauma grave, que apresentou trombose arterial em perna direita 24 horas após a queimadura. Paciente submetido à amputação do membro e a seriadas intervenções cirúrgicas para desbridamento e enxertia de pele. Apesar dos protocolos de prevenção, paciente foi infectado pela COVID-19 durante a internação.
Conclusão: Este caso enfoca uma complicação rara relacionada à lesão por queimadura, que ainda não possui critérios de diagnóstico e medidas profiláticas definidas. Além disso, a pandemia de COVID-19 trouxe impactos em diversos âmbitos nos serviços de saúde, sendo fundamental o compartilhamento de conhecimentos durante esta pandemia pela busca de adaptações à situação de crise.
Palavras-chave: Queimaduras; Traumatismo múltiplo; Trombose; Coagulação intravascular disseminada; Vírus da SARS.
Introduction
On March 11, 2020, the COVID-19 pandemic was declared, caused by the new coronavirus. On March 30, 2020, when 320 cases had been registered in Brazil, the Division of Plastic Surgery and Burns of the largest hospital complex in Latin America was physically aimed at the infected; burned patients were transferred to another burn center, and health professionals were relocated.
In this context, we had the challenge of treating a large burned and multiple trauma patient with arterial thrombosis in the right lower limb, a rare complication associated with the burn. His treatment was carried out in one of the complex’s institutes, which is not specialized in trauma care, but where the structure was temporarily moved to these services, and where he remained hospitalized due to the need for specialized multidisciplinary care.
CASE REPORT
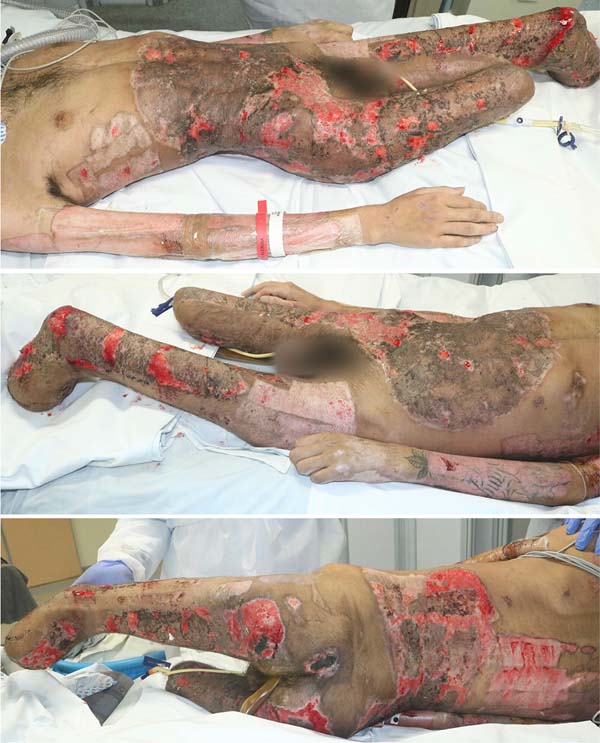
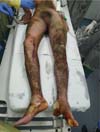
An 18-year-old male patient, without comorbidities, truck collision victim, followed by an explosion of a gasoline tank on his motorcycle, had predominantly 3rd degree burns with an estimated burned body surface (BBS) of 50% in the lower limbs, abdomen, lower back and left forearm, and second-degree burns on the right forearm (Figure 1).
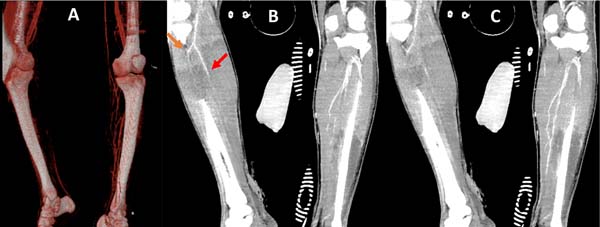
Computed tomography (CT), subarachnoid hemorrhage, hemoventriculus (IV ventricle) and signs of diffuse axonal lesion are identified. On CT control, there were signs of worsening of cerebral edema; we opted for external ventricular drain (EVD) and passage of intracranial pressure catheter (ICP) by the neurosurgery team.
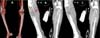
After 24 hours, he presented edema, decreased temperature and non-fixed cyanosis of the right lower limb with little improvement of possible venous congestion post-escharotomy. After 12 hours with worsening of the perfusion, he was submitted to angiotomography (CTA) (Figure 2), evidencing filiform flow and sometimes an absence of flow in the anterior and posterior tibial artery, tibiofibular trunk, fibular and difficult characterization in the more distal segments, evolving with fixed cianosis. Because of vascular injury’s situation not yet delimited, extensive deep burn and severe neurotrauma, amputation after clinical compensation was chosen.
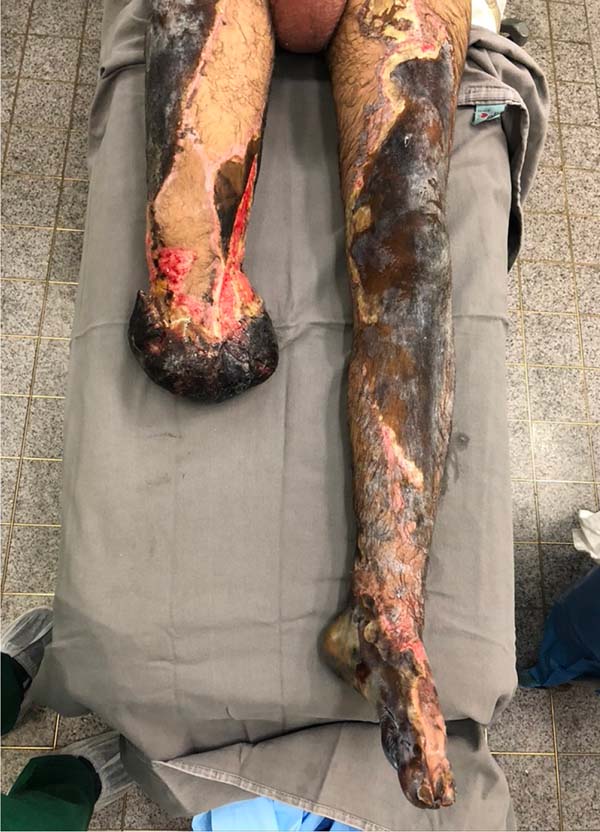
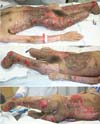
After the withdrawal of EVD and ICP with an improvement of the clinical picture, open transtibial amputation was performed on the 13th day of hospitalization. After six days, the stump was ischemic with necrosis (Figure 3), requiring enlargement of the amputation level to transfemoral.
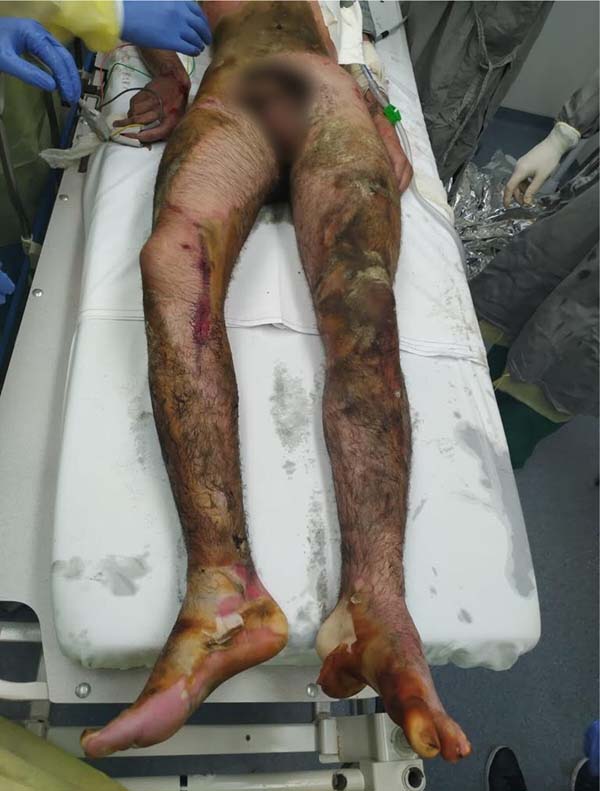
From there, we perform weekly serial operations with debridement and mesh skin grafting. Table 1 summarizes our surgical interventions. Neurologically, the patient showed improvement in the level of consciousness and motor coordination, also having areas of ulceration (Figure 4) for future grafting.
In the 27th IHL, molecular test (RT-PCR) was collected for COVID-19, which had been negative, for investigation of febrile peaks without other symptoms, treated with antibiotic therapy by bloodstream infection. In the 41st IHL, a COVID-19 serological test was performed from all hospitalized patients in the same intensive care unit (ICU) due to one of the patients’ infections. In this, there was positivity for immunoglobulin G.
| #Surgery - Day of hospitalization (IHL) | Evolution |
|---|---|
| #1 - 2nd IHL EVD by the neurosurgery team. | Withdrawal at 5º IHL after intracranial pressure remains normal with CLOSED EVD for 72 hours. |
| #2 - 12th IHL Right open transtibial amputation by vascular surgery (VC): the muscle presented little bleeding, presence of hematomas, thrombosed vessels; it was then decided to keep open muscle flap without skin coverage to evaluate viability, and installed subatmospheric pressure therapy (VAC). | VAC dressing removed after 6 days showing muscle stump flap with necrosis and ischemic aspect (Figure 2). |
| #3 - 18th IHL Right transfemoral amputation by VC. Debridement and grafting on the abdomen and left forearm. | Opening of graft dressing after 6 days: integration >90%. |
| #4 - 24th IHL Left transtibial amputation by VC due to the extent and depth of the burn in the foot, leading to the unviability of limb reconstruction. Debridement and grafting on the right transfemoral stump. | Opening of graft dressing after 6 days: integration >90%. |
| #5 - 31st IHL Debridement and grafting on the back, inguinal region and gluteus on the right. | Opening of graft dressing after 7 days: integration ~60%. |
| #6 - 32nd IHL Tracheostomy by thoracic surgery for airway protection after extubation failure (neurological status + psychomotor agitation). | |
| #7 - 38th IHL Debridement and grafting on the left stump. | Opening of graft dressing after 7 days: integration ~80%. |
| #8 - 45th IHL Debridement and grafting on the back and glutes. | Opening of graft dressing after 7 days: integration ~80%. |
DISCUSSION
Extensive burn is one of the most severe and complex forms of trauma1. In this case, there was a beneficial sharing of the assistance team’s performance of the non-specialized institute with the team of the burn division. There was a need for nursing, not affected by the treatment of burn patients, to be quickly trained in applying the dressings, and joint efforts with the plastic surgery team to integrate the grafts and restore the donor area (reused after 15 days) were essential.
Early activation of uncontrolled coagulation and fibrinolysis may be consequences of endothelial injury and systemic inflammatory response2. Current evidence suggests that the uncontrollable increase in the coagulation system is proportional to BBS, burn depth, inhalation injury, and hemodilution in the volume resuscitation phase3.
A severe burn can cause thrombosis of capillaries and small-caliber veins, but rarely medium and large caliber4.5. Few cases of burn-induced arterial thrombosis have been reported, although well described in necropsies of burned patients5. Among these cases, most patients had risk factors such as atherosclerotic disease or artery catheterization6. In this case, the patient without comorbidities presented early laboratory alterations (Table 2) associated with hemoconcentration with increased hemoglobin and hematocrit (increased blood viscosity, risk factor for thrombosis); blood dyscrasia with increased prothrombin time (PT) and activated partial thromboplastin time (aTTP) and platelet intake, suggesting disseminated intravascular coagulation (IVC). In the IVC, there is a continuous procoagulant activity with fibrin deposition in small and medium vessels that consume and deplete coagulation factors, which may cause thrombosis and bleeding7. Kinetic studies with fibrinogen and marked platelets reveal that, in addition to this systemic consumption, significant local consumption occurs in burned areas8. In this case, CTA suggested native arterial thrombus, which could be caused by atherosclerosis, aneurysm, dissection or hypercoagulability9. The trauma could lead to arterial thrombosis subsequent to a pseudoaneurysm or arterial dissection, which was not seen on imaging10. Thus, the most likely etiology of thrombosis was due to hypercoagulability associated with IVC secondary to the large burned systemic inflammatory response.
Neurotrauma11 contraindicated the use of thrombolytics, and embolectomy was deprecated, as it would need to be performed early when the patient still needed clinical and neurological compensation. For this reason, and the depth and extent of the burns, we opted for limb amputation.
| Hb (g/dL)/Ht (%) | Platelets | INR | ATTP | CRP | |
|---|---|---|---|---|---|
| 11/04/20 | 19.9 / 55 | 565.000 | 1.6 | 1.3 | |
| 12/04/20 | 23 / 65 | 309.999 | 1.9 | 1.17 | 39 |
| 13/04/20 | 20.0 / 61 | 183.000 | 2.2 | 1.39 | 117 |
| 14/04/20 | 12.2 / 35 | 76.999 | 1.5 | 1.22 | 87.68 |
| 15/05/20 | 11.6 / 32 | 77.000 | 1.2 | 1.2 | 103.54 |
Despite the absence of local infectious complications, we considered the surgical approach late. In addition to waiting for better clinical conditions, the physical loss of our specialized structure for burns caused logistical difficulties, from obtaining an operating room to the availability of our specific equipment and supplies. Fortunately, after the reorganization of the hospital complex, we were able to perform weekly surgical interventions.
To prevent COVID-19, patients hospitalized were allocated according to their infectious status, with wards for suspects, infected and uninfected. Every patient treated in the emergency room performs RT-PCR infectious screening and is considered suspicious, remaining in isolation with droplet precaution. After the result, the patient is referred to his/her respective ward, where constant surveillance of infectious signs (fever and respiratory symptoms) is performed. If present, they are re-transferred to the suspect ward until thorough investigation with imaging and new RT-PCR for COVID-19. Besides, there was an impediment to any patient’s visitation, either in the ward or ICU. Despite these efforts, the patient was infected during hospitalization and, fortunately, did not present any respiratory complication or other thrombotic complication.
CONCLUSION
Despite the knowledge of burn-related coagulopathy, we still need evidence of diagnostic criteria, prophylaxis and treatment. COVID-19 caused important changes in our clinical-surgical practice. Thus, the sharing of knowledge and skills, interaction between professionals from various areas, and the search for solutions adapted to the crisis situation can emerge as a positive legacy in the pandemic face.
REFERENCES
1. Zuo KJ, Medina A, Tredget EE. Important developments in burn care. Plast Reconstr Surg. 2017 Jan;139(1):120e-38e. DOI: https://doi.org/10.1097/PRS.0000000000002908
2. Glas GJ, Levi M, Schultz MJ. Coagulopathy and its management in patients with severe burns. J Thromb Haemost. 2016 Mai;14(5)865-74.
3. Marsden NJ, Van M, Dean S, Azzopardi EA, Hemington GS, Evans PA, et al. Measuring coagulation in burns: an evidence-based systematic review. Scars Burn Heal. 2017 Set;3:2059513117728201. DOI: https://doi.org/10.1177/2059513117728201
4. Counce JS, Cone JB, McAlister L, Wallace B, Caldwell Junior FT. Surgical complications of thermal injury. Am J Surg. 1988 Dez;156(6):556-7. DOI: https://doi.org/10.1016/s0002-9610(88)80552-x
5. Sevitt S. A review of the complications of burns, their origin and importance for illness and death. J Trauma. 1979 Mai;19(5):358-69. DOI: https://doi.org/10.1097/00005373-197905000-00010
6. Barret JP, Dziewulski PG. Complications of the hypercoagulable status in burn injury. Burns. 2006;32(8):1005-8. DOI: https://doi.org/10.1016/j.burns.2006.02.018
7. Pintão MCT, Franco RF. Coagulação intravascular disseminada. Medicina (Ribeirão Preto). 2001 Dez;34:282-91.
8. Levi M, Van Der Poll T. Disseminated intravascular coagulation: a review for the internist. Intern Emerg Med. 2013 Set;8(1):23-32. DOI: https://doi.org/10.1007/s11739-012-0859-9
9. Leung LLK. Clinical features and diagnosis of acute lower extremity ischemia. Waltham, MA: UpToDate; 2020.
10. Mitchell ME, Carpenter JP. Clinical features and diagnosis of acute lower extremity ischemia. Waltham, MA: UpToDate; 2020.
11. Braun JD. Embolism to the lower extremities. Waltham, MA: UpToDate; 2020.
1. Hospital das Clínicas, Faculty of
Medicine, University of São Paulo, Department of Plastic Surgery and
Burns, São Paulo, SP, Brazil.
Corresponding author: Rafael Eiki Takemura, Avenida Doutor Enéas Carvalho de Aguiar, 255, Cerqueira César, São Paulo, SP, Brazil., Zip Code: 05403-000, E-mail: eikitakemura@gmail.com
Article received: July 06, 2020.
Article accepted: January 10, 2021.
Conflicts of interest: none
Institution: Hospital das Clínicas, Faculty of Medicine, University of São Paulo, Department of Plastic Surgery and Burns, São Paulo, SP, Brazil.






 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter