

Case Report - Year 2021 - Volume 36 -
Use of thoracoepigastric flap in the closure of large chest wall defects after surgical treatment of locally advanced breast tumor: a case report
Uso do retalho toracoepigástrico no fechamento de grandes defeitos na parede torácica pós-tratamento cirúrgico de tumor de mama localmente avançado: relato de caso
ABSTRACT
Breast cancer is the most common type of malignancy among women in Brazil and worldwide, excluding non-melanoma skin cancers. The purpose of this report is to describe the case of a patient with invasive breast carcinoma, associated with a large extent of skin involvement and nipple-areola complex, whose lesion was unchanged after neoadjuvant chemotherapy. After a Halsted mastectomy, the thoraco-epigastric flap was used to close the thoracic defect, with a favorable evolution of the patient. The use of the thoraco-epigastric flap has been described as a reliable tool because it is characterized as a technique that is easy to perform, safe and with minimal post-surgical complications.
Keywords: Breast neoplasm; Radical mastectomy; Myocutaneous flap; Neoadjuvant therapy; Surgical oncology.
RESUMO
O câncer de mama é o tipo de neoplasia maligna mais comum entre as mulheres no Brasil e no mundo, excluindo-se as neoplasias de pele não melanoma. O objetivo do presente relato é descrever o caso de uma paciente portadora de carcinoma invasor da mama, associado a grande extensão de comprometimento de pele e complexo aréolomamilar, cuja lesão mostrou-se inalterada após quimioterapia neoadjuvante. Após mastectomia tipo Halsted, utilizou-se o retalho tóraco-epigástrico para fechamento do defeito torácico, com evolução favorável da paciente. O uso do retalho tóracoepigástrico tem sido descrito como uma ferramenta confiável por caracterizar-se como uma técnica de fácil execução, segura e com mínimas complicações pós-cirúrgicas.
Palavras-chave: Neoplasia de mama; Mastectomia radical; Retalho miocutâneo; Terapia neoadjuvante; Oncologia cirúrgica.
INTRODUCTION
According to the National Cancer Institute, breast cancer is the most common type of malignancy among women in Brazil and worldwide, excluding non-melanoma skin neoplasms, with 1.2 million new cases diagnosed annually, totaling 15% of all cancer deaths in women1-3. In Brazil, this percentage is 29%, and for the year 2020, 66,280 new breast cancer cases are expected1,3-6.
Despite prevention campaigns, locally advanced breast cancer, not being the majority of cases, can cover up to 50% of cases7. Due to its severity, the initial therapeutic approach has been using neoadjuvant chemotherapy for possible primary reduction of the lesion and subsequent surgical treatment. In resistant cases, mastectomy becomes the treatment of choice, including axillary emptying and large skin resections, leading to important chest wall defects7.
The thoracoepigastric flap has been an important surgical tool for such cases, with an excellent prognosis and low rate of complications3.
The case of a patient with locally advanced breast cancer, associated with extensive involvement of the skin and pectoral muscles, with a large defect of the chest wall after surgical removal of the tumor is described, and the thoracoepigastric flap technique was used for complete closure of the region.
CASE REPORT
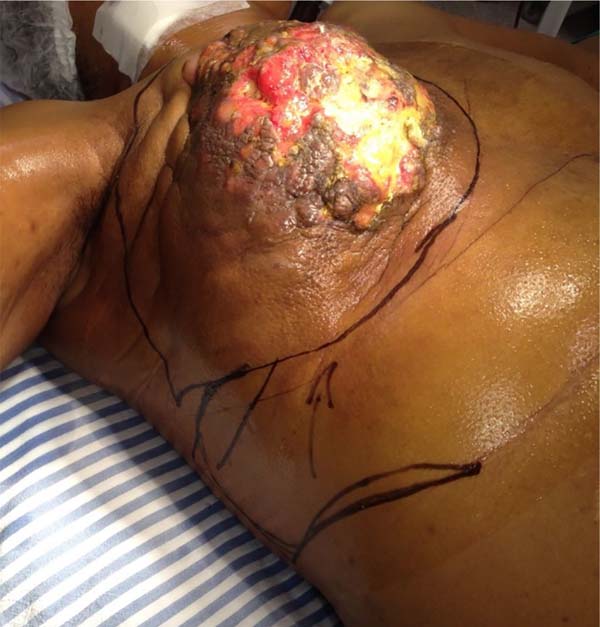
R.A.S., female, 53 years old, from Regent Feijó/SP, with no family history of breast cancer, sought her municipality’s health center, stating that six months ago, she noticed a nodule in her right breast during the self-examination. She was referred to the Mastology outpatient clinic of the Regional Hospital of Presidente Prudente, presenting, on physical examination, a voluminous tumor occupying the entire length of the right breast, measuring approximately 20cm in diameter, fixed to the deep planes, invading the skin, providing ulceration and destruction of the nipple-areolar complex. Biopsy showed infiltrating mixed carcinoma (lobular component with discrete areas of non-special ductal carcinoma).
The patient underwent neoadjuvant chemotherapy treatment with four cyclophosphamide doxorubicin cycles, followed by 12 paclitaxel cycles, without the lesion’s clinical regression.
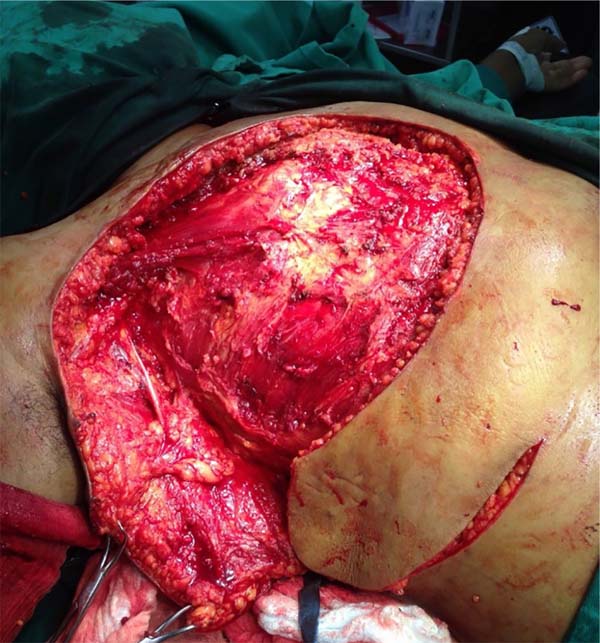
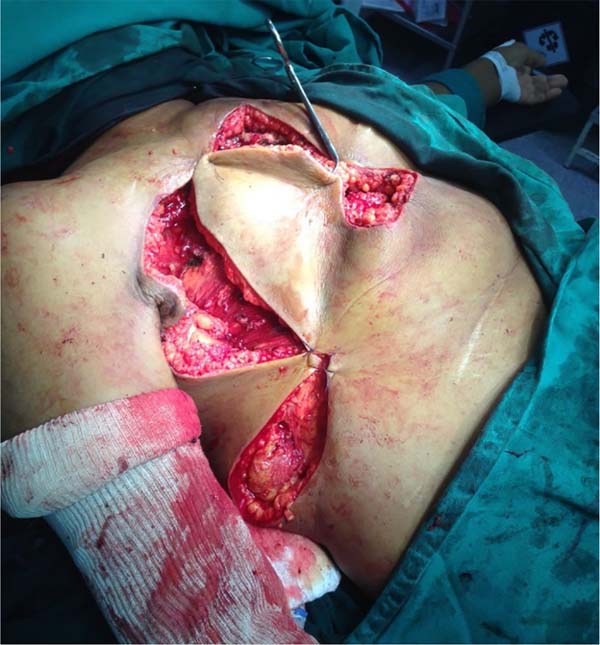
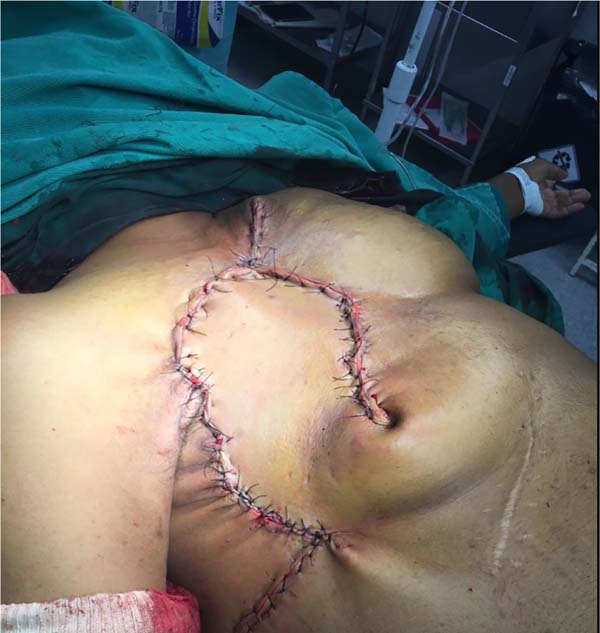
She was referred for surgical treatment, in which a mastectomy was performed with subsequent closure of the chest wall defect using thoracoepigastric flap. The technique involved the following steps: a) with the patient in a supine position, surgical marking of the area to be resected and the flap was performed (Figure 1); b) a wide resection of the tumor area was carried out, including breast, pectoral musculature and adjacent cutaneous tissue, with subsequent axillary emptying; c) making the flap by detaching it from the abdominal wall, keeping it pedicled in the epigastric region (Figure 2); c) rotation and fixation of the flap in the defective area (Figure 3); d) approximation and synthesis of local flaps with complete closure of the thoracic defect (Figure 4).
The patient progressed favorably with good flap perfusion, without areas of necrosis or infection. The surgical specimen’s anatomopathological analysis confirmed the diagnosis of mixed carcinoma measuring 16x14cm of high degree. It was observed that the tumor mass invaded the pectoral muscle and with great involvement of the skin and papilla.
DISCUSSION
Locally advanced breast tumors are defined as a neoplasm that compromises the breast in all, or almost all of its extension, tumors that compromise four or more axillary lymph nodes, or those with metastases in ipsilateral supraclavicular lymph nodes4,7.
According to Ho et al., in 20168, its treatment should include loco-regional disease control and eradicating occult systemic metastases. In the case of voluminous tumors requiring extensive skin losses, impossible to be reparated with primary closure, several studies highlight the importance of using oncoplastic techniques2,4,5,7-9. There is no established consensus on the best approach to be defined according to the surgeon’s experience and preference and the quality of tissue adjacent to the breast and intraoperative factors of the patient3-5,7.
The flaps appeared in 1886, with Tansini, and among the existing options, stand out the large dorsal muscle, the transverse flap of the rectus abdominal muscle (TRAM) or vertical flap rectus abdominal muscle (VRAM), the thoracoepigastric or thoracoabdominal flap and skin grafts2-5,7,10.
The use of the thoracoepigastric flap has been described as a reliable tool because it is characterized as a technique that is easy to perform, safe and with minimal post-surgical complications, highlighting the cases in which it is desired that the radiotherapy and chemotherapy treatment should not be delayed5,7. Initially described in 1974 by Bohmert e Cronin, in 198011, this method allows the coverage of extensive defective areas of the breast, in lower or lateral thoracic regions, in addition to sternal defects, without the need for other flaps or skin grafts11,12. Park et al., in 200613, described a series of 24 cases in which the closure of a large compromised area after mastectomy was performed with thoracoepigastric fasciocutaneous flaps, which were safe with 36% of small complications, possible to be recovered with conservative treatment, in a14-month follow-up13. Davis et al., in 197714, describe16 other cases with the use of the thoracoepigastric flap, which did not present complications, so they are shown to be very safe and effective techniques14.
The thoracoepigastric flap derives from a richly vascularized region with superficial segmental blood supply of perforating arteries, which allows the manufacture of long resistant and safe fragments4,6. They are designed as transposition flaps, and the determination of length remains uncertain6. Davis et al., in 197714, reported the largest flap size being 35x15cm2. In this case, we used a fabric fraction of 15x8cm, ensuring the necessary coverage of the existing defect14.
There are numerous advantages described in the use of thoracoepigastric flaps, such as shorter surgical time compared to other techniques, less blood loss and less postoperative hospital stay4. According to Deo et al., in 200315, patients who underwent musculocutaneous reconstructions demonstrated increased morbidity, blood loss in the abdominal wall and prolonged hospital stay compared to thoracoabdominal reconstructions15,16.
CONCLUSION
It is concluded that this technique is extremely useful, innovative and easily executable. The use of thoracoepigastric flap represents an important tool in closing chest wall defects, with satisfactory results.
REFERENCES
1. Ministério da Saúde (BR). Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Estimativa 2018: incidência de câncer no Brasil. Rio de Janeiro: Ministério da Saúde/INCA; 2017.
2. Cosac OM, Camara Filho JPP, Cammarota MC, Di Lamartine J, Daher JC, Borgatto MS, et al. Reconstrução mamária de resgate: a importância dos retalhos miocutâneos. Rev Bras Cir Plást. 2013;28(1):92-9.
3. Fitoussi AD, Berry MG, Famà F, Falcou MC, Curnier A, Couturaud B, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases. Plast Reconstr Surg. 2010 Fev;125(2):454-2.
4. Saldanha OR, Urdaneta FV, Llaverias F, Saldanha Filho OR, Saldanha CB. Reconstrução de mama com retalho excedente de abdominoplastia reversa. Rev Bras Cir Plást. 2014;29(2):297-302.
5. Pinto EBS, Muniz AC, Erazo PI, Abdalla PCSP. Reconstrução mamária: princípios geométricos dos retalhos cutâneos em duplo V. Rev Bras Cir Plást. 1998;13(3):19-42.
6. Pires DM, Souza GJ, Pace B. Utilização do retalho tóraco-epigástrico em grandes ressecções da mama: relato de caso de tumor filóide. Mastology (Impr.). 2018 Dez;28(4):231-5.
7. Burattini ACB, Piteri RCO, Ferreira LF, Silveira Junior VF, Broetto J, Richter CA, et al. Segurança e viabilidade de um novo formato de retalho toracoepigástrico na reconstrução da parede torácica em câncer de mama localmente avançado: um estudo transversal. Rev Bras Cir Plást. 2016;31(1):2-11.
8. Ho W, Stallard S, Doughty J, Mallon E, Romics L. Oncological outcomes and complications after volume replacement oncoplastic breast conservations—the Glasgow experience. Breast Cancer (Auckl). 2016;10:223-8.
9. Cammarota MC, Campos AC, Faria CADC, Santos GC, Barcelos LDP, Dias RCS, et al. Qualidade de vida e resultado estético após mastectomia e reconstrução mamária. Rev Bras Cir Plást. 2019;34(1):45-57.
10. Marcondes CA, Pessoa SGP, Pessoa BBGP, Dias IS, Ribeiro NP. Estratégias em reconstruções de tórax pós ressecções extensas de tumores de mama localmente avançados: uma série de 11 casos. Rev Bras Cir Plást. 2015;30(13):339-44.
11. Bohmert H. Experience in breast reconstruction with thoraco-epigastric and advancement flaps. Acta Chir Belg. 1980;79(2):105-10.
12. Cronin TD, Upton J, McDonough JM. Reconstruction of the breast after mastectomy. Plast. Reconstr. Surg. 1977; 59:1
13. PARK P. et al. Extended cutaneous ‘thoracoabdominal’ flap for large chest wall reconstruction. Ann Plast Surg. 2006;57(2):177-83.
14. Davis WM. et al. Use of a direct, transverse, thoracoabdominal flap to close difficult wounds of the thorax and upper extremity. Plast Reconstr Surg. 1977;60(4):526-33. PMID: 333484 DOI: http://dx.doi.org/10.1097/00006534-197710000-00005
15. Deo S. V. et al. Myocutaneous versus thoraco-abdominal flap cover for soft tissue defects following surgery for locally advanced and recurrent breast cancer. J Surg Oncol. 2003;83:31-35.
16. Silva MMA, Sabino Neto M, Leite AT, Guimarães PAP, Ferreira LM. Reconstrução de parede torácica em extensas ressecções oncológicas. Rev Bras Cir Plást. 2017;32(4):513-22.
1. Universidade do Oeste Paulista, Faculdade de
Medicina de Presidente Prudente, Presidente Prudente, SP,
Brazil.
Corresponding author: Rafaela Alias Horta, Rua Rio Grande do Sul, 244, Vila Marcondes, Presidente Prudente, SP, Brazil., Zip Code: 19030-130, E-mail: aliasrafaela@gmail.com
Article received: July 17, 2020.
Article accepted: January 10, 2021.
Conflicts of interest: none
Institution: Universidade do Oeste Paulista, Faculdade de Medicina de Presidente Prudente, Presidente Prudente, SP, Brazil.




 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter