

Original Article - Year 2021 - Volume 36 -
Infiltration of local anesthetics into the surgical wound: effect on inflammation and fibrous scar in rats
Infiltração de anestésicos locais na ferida cirúrgica: efeito sobre a inflamação e cicatriz fibrosa em ratos
ABSTRACT
Introduction: Pain relief after surgery remains one of the most significant medical challenges, mainly in aesthetic surgery. The infiltration of the surgical incision with local anesthetics has been increasingly used to reduce pain and other analgesic use. However, little is known about the effect of this injection on healing. The objective is to evaluate the interference of local anesthetics in the area of inflammatory infiltrate and fibrosis scar in rats.
Methods: Two linear incisions each were made on the dorsal region of 40 Wistar rats. The left incision was infiltrated with doses of 1.8ml of bupivacaine, levobupivacaine, ropivacaine, or 0,9% saline solution infiltration. The right incision did not receive infiltration, serving as a control group. After seven days, samples of the incisions were collected for histological morphometric evaluation.
Results: When compared with the control groups, the area of inflammatory infiltrate was found larger in the bupivacaine, ropivacaine, and levobupivacaine groups. The bupivacaine group presented a larger inflammatory infiltrate than the levobupivacaine and ropivacaine. The fibrous scar area was larger in the levobupivacaine and ropivacaine groups. There was no difference between the groups that received anesthetic and saline solution.
Conclusion: As there was no difference between the anesthetics and saline solution groups, the volume applied, or the trauma may have been the cause of the larger areas of infiltrating and scar associated with local anesthetics application.
Keywords: Inflammation; Bupivacaine; Levobupivacaine; Ropivacaine; Cicatrix.
RESUMO
Introdução: O alívio da dor após a cirurgia continua sendo um dos desafios médicos mais significativos, principalmente na cirurgia estética. A infiltração da incisão cirúrgica com anestésicos locais tem sido cada vez mais utilizada para reduzir a dor e o uso de analgésicos. No entanto, pouco se sabe sobre o efeito desta injeção na cicatrização. O objetivo é avaliar a interferência dos anestésicos locais na área de infiltrado inflamatório e cicatriz de fibrose em ratos.
Métodos: Duas incisões lineares foram feitas cada uma na região dorsal de 40 ratos Wistar. A incisão esquerda foi infiltrada com doses de 1,8ml de bupivacaína, levobupivacaína, ropivacaína ou solução salina 0,9%. A incisão direita não recebeu infiltração, servindo como grupo controle. Após sete dias, amostras das incisões foram coletadas para avaliação morfométrica histológica.
Resultados: Quando comparada com os grupos controle, a área de infiltrado inflamatório encontrada foi maior nos grupos bupivacaína, ropivacaína e levobupivacaína. O grupo bupivacaína apresentou um infiltrado inflamatório maior do que a levobupivacaína e a ropivacaína. A área da cicatriz fibrosa foi maior nos grupos levobupivacaína e ropivacaína. Não houve diferença entre os grupos que receberam anestésico e solução salina.
Conclusão: Como não houve diferença entre os grupos de anestésico e soro fisiológico, o volume aplicado ou o trauma podem ter sido a causa das maiores áreas de infiltração e cicatriz associadas à aplicação dos anestésicos locais.
Palavras-chave: Inflamação; Bupivacaína; Levobupivacaína; Ropivacaína; Cicatriz.
INTRODUCTION
Pain relief after surgery remains one of the most significant medical challenges, and inadequate treatment may delay hospital discharge and patient recovery. In addition, postoperative pain is acute and, when improperly conducted, may lead to the development of chronic pain and greater use of analgesics, including opioids, and their consequences1,2.
The infiltration of surgical incisions with local anesthetics (LA), mainly those long-lasting, has been increasingly used in different types of surgeries, and the results described in studies are encouraging1,2. The effects of this application have been tested in several experimental models3-7. However, the impact of LA infiltration on surgical incision healing has not yet been fully established.
Aesthetic surgeries sometimes are significant, and consequently, there may be severe pain in the postoperative period. The infiltration of LA could improve this pain; however, it is necessary to assess whether this infiltration influences healing, which could harm the final result.
OBJECTIVE
This study aimed to evaluate the interference of the infiltration of long-lasting LAs in the surgical incision on the inflammatory infiltrate and on the fibrous scar areas in rats.
METHODS
This study was carried out in a public tertiary hospital (Hospital de Clínicas, Universidade Federal do Triângulo Mineiro, Uberaba, Minas Gerais, Brazil) from January 2018 to January 2020 approved by the committee on ethics in animal use under protocol number 314. All the animals were treated following the recommendations of the institutional animal care committee.
We followed the committee’s recommendations on ethics in animal use to comply with the principle of 3R (reduction, refinement, and replacement). No sample calculation was performed. Forty Wistar rats, 20 males and 20 females, were divided into four groups of 10, with the same number of females and males in each group. The animals did not present a difference in their weight, ranging from 152 to 378 grams, with an average of 264 grams (±0.06).
The rats were placed in cages with controlled temperature (24±1°C) and in cycles of 12 hours with light-12 hours in the dark. They were fed a standard diet with water ad libitum for 12 hours before the experimental protocol.
The surgical procedure was performed under general anesthesia, induced by xylazine hydrochloride 2%, 5mg/kg, associated with ketamine, 70mg/kg, administered intraperitoneally. When anesthesia was confirmed (loss of tail reflex, paws and muscle relaxation), the dorsal area of the animal was trichotomized, cleaned with povidone- iodine and dried with sterile gauze after 2 minutes. Two linear incisions of about 2cm each were made on the dorsal region, symmetrical concerning the midline, reaching the subcutaneous tissue. The left incision was infiltrated with doses of 1.8ml of LA (Figure 1), and the right incision did not receive infiltration (control group). Another group received 1,8ml of 0.9% saline solution (SS) application in the left incision and nothing in the right. Thus, six application points (0.3ml per point) distributed along the 2cm incision were made.
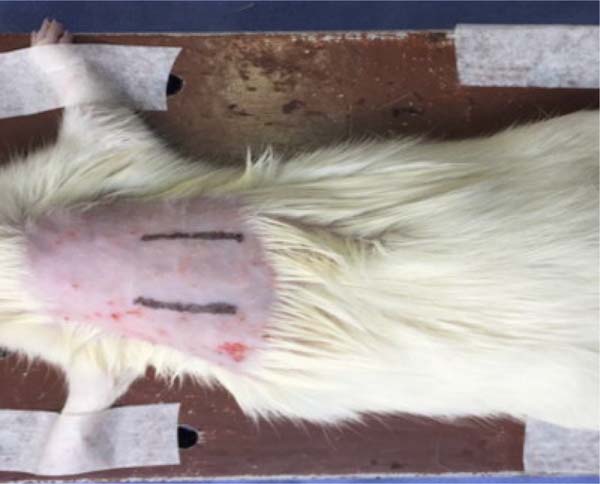
The groups were named according to the substance applied in the incision:
There was no evaluation of anesthetic equipotency. Therefore, we chose to standardize the volume and number of application points in the groups.
Two minutes after infiltration, the incisions were sutured using a 4.0 prolene suture thread. There was an observation period of 2 hours after the application of the local anesthetic. The test animals received an intramuscular tramadol injection (2mg/kg) during the postoperative period every 12 hours for three days. The surgical wound was treated once a day, and the animals were sacrificed at the end of the seventh day.
Immediately after sacrifice, tissue samples from the two incisions with an average diameter of 2cm2 were removed and fixed in 4% buffered formaldehyde for approximately 4 hours. Posteriorly, they were cleaved, and samples of the scar were processed and embedded in paraffin to make histological sections of about 5 micrometers (µm) thick. Next, histological sections were stained using the hematoxylin-eosin (HE) techniques to evaluate the inflammatory infiltrate and the Masson’s trichrome (MT) staining for the evaluation of the fibrous scar. The slides were analyzed under a standard light microscope (OLYMPUS® BX40) by a single previously trained observer, who was unaware of the group to which each animal belonged (blind).
To perform the morphometric analysis of the area of the inflammatory infiltrate (HE) and the fibrous scar (MT), we selected the areas with the largest inflammatory infiltrate around the surgical scar (HE) and the focus with the largest scar diameter (MT), respectively. To determine the area of the inflammatory infiltrate and the scar, Image J® 1.32j semiautomatic image analyzer system (National Institutes of Health, U.S.) was used, using µm as reference unit and the 100X magnification field of light microscope Olympus BX41 (Olympus®, São Paulo, Brazil).
Biostat® program, version 5.0, was used for statistical analysis. Comparisons among the different groups were made using a one-way ANOVA test with Bonferroni post-test and Kruskal-Wallis test with Student-Newman-Keuls post-test. The results were considered significant when the probability of rejection of the null hypothesis was less than 5% (p<0.05).
RESULTS
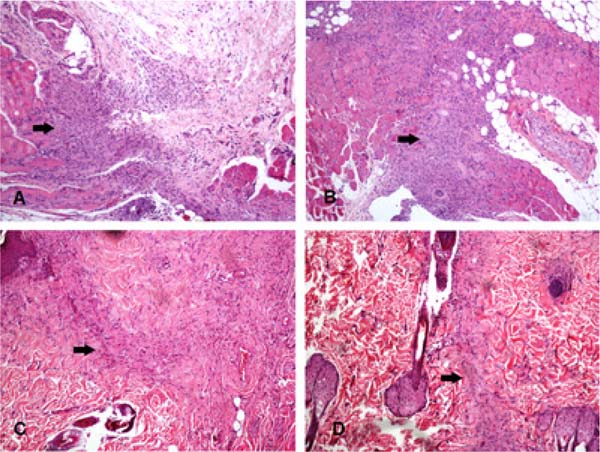
Morphologically evaluating, the inflammatory infiltrate was predominantly mononuclear, permeated by occasional polymorphonuclear cells, especially neutrophils. Table 1 shows the results of the inflammatory infiltrate area in the different groups.
The inflammatory infiltrate area was significantly larger in the bupivacaine, ropivacaine, and levobupivacaine groups compared with their controls (incisions that did not receive any infiltration). When comparing the groups with each other, the bupivacaine group had a significantly larger inflammatory infiltrate than the levobupivacaine and ropivacaine groups. In addition, the SS 0.9% group presented a substantially larger area of infiltrating than its control group. However, no difference was observed between the SS 0.9% group and the LA groups tested.
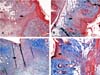
Figure 2 illustrates the inflammatory infiltrate area in four different animals. Table 2 shows evaluation data of the fibrous scar area.
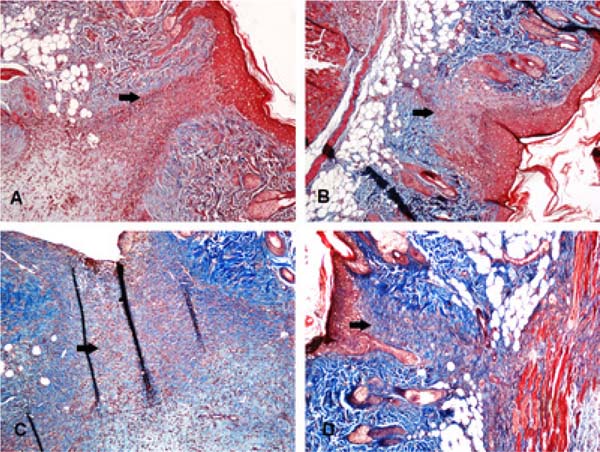
When evaluating the fibrous scar area, a significant difference was observed between the levobupivacaine and ropivacaine groups and their controls. Moreover, the fibrous scar area was larger when these two anesthetics were applied. However, when comparing the LA groups with the SS 0.9% group, no difference was found.
Figure 3 shows the fibrous scar in four different animals.
DISCUSSION
LA infiltration into the skin and subcutaneous tissue is widely used for analgesic purposes after surgical correction in different types of surgery1,2. Long-lasting LAs, such as bupivacaine, ropivacaine, and the enantiomeric excess levobupivacaine, corresponds to 75% of the levorotatory isomer and 25% of the dextrorotatory isomer are regularly used. Theoretically, the infiltration of these anesthetics is the most rational method of analgesia since they block the nociceptive afferents and, therefore, pain and secondary stress8-10.
There has been controversy over the interference of LA in the healing of surgical wounds. The effects of injection or topical application of LA have been tested in several experimental models, using healing time, tensile strength measures, inflammatory process area, regeneration, granulation tissue, and angiogenesis3-7. These studies used rodents whose skin heals rapidly, less susceptible to inhibitory agents and systemic factors that may limit healing3.
The healing of surgical wounds is when the tissue at the lesion site is replaced by vascularized connective tissue. The first healing step is the onset of an inflammatory reaction that will reabsorb extravasated blood and tissue degradation products. Subsequently, there is a proliferation of capillaries and fibroblasts that will form the cicatricial connective tissue. The final volume of the scar depends on the stimuli that regulate the activity of the cells that produce the extracellular matrix and on balance between the matrix synthesis and degradation11. There is evidence that the intensity and type of the inflammatory infiltrate will define the extent of the scar12. In the present study, the evaluation was carried out seven days after the incision. At this time, healing is probably in the granulation/proliferation stage, which is characterized by fibroblast migration and collagen production in rats13. However, even in this stage, significant inflammatory infiltration around the scar was observed in all groups.
| Animal Group | Median area (µm2) | Minimum area (µm2) | Maximum area (µm2) |
|---|---|---|---|
| Bupivacaine | 1595646.75a | 353178.5 | 3785935.5 |
| Bupivacaine control | 10301715 | 181919 | 2865686.5 |
| Levobupivacaine | 975600b.e | 661763 | 1829388 |
| Levobupivacaine control | 762221.8 | 97105 | 971325 |
| Ropivacaine | 1278801.3c.f | 379043.5 | 4122721.5 |
| Ropivacaine control | 340497 | 340497 | 565769 |
| SS 0.9% | 1440765.3d | 832288 | 1959811 |
| SS 0.9% control | 749901.3 | 74383 | 1054869 |
Control group: incision that did not receive infiltration.
a Bupivacaine x Bupivacaine control p<0,05;
b Levobupivacaine x Levobupivacaine control p<0,05;
c Ropivacaine x Ropivacaine control p<0,05;
d SS x SS control p<0,05; eBupivacaine x Levobupivacaine p<0,05;
f Bupivacaine x Ropivacaine p<0,05; One-way ANOVA. SS: Saline Solution.
| Animal Group | Median area (µm2) | Minimum area (µm2) | Maximum area (µm2) |
|---|---|---|---|
| Bupivacaine | 449402 | 146280 | 2016103.5 |
| Bupivacaine control | 605389.5 | 47813.5 | 1251420.5 |
| Levobupivacaine | 818835.5a.c | 346804 | 4741842 |
| Levobupivacaine control | 450434.0 | 116934 | 779886 |
| Ropivacaine | 885409b.d | 295817 | 2599696 |
| Ropivacaine control | 321682 | 207731 | 725589 |
| SS 0.9% | 530607.8 | 235281.5 | 1997949 |
| SS 0.9% control | 501198.5 | 140366 | 1089956 |
Levobupivacaine was associated with the smallest area of inflammatory infiltrate and one of the largest areas of a fibrous scar. This observation contradicts the conception that the intensity of the inflammatory infiltrate defines the extent of scar formation12. It is possible that the type of inflammatory infiltrate, rather than its intensity, may be associated with a larger or smaller fibrous scar area, which was not evaluated in the current study. The presence of inflammatory infiltrate interferes directly in the scar formation; however, different types of inflammatory cells may be present, and these cells may, or not, stimulate the production of extracellular matrix. Another possibility would be that the volume or trauma of the application, not the LA itself, would interfere with the inflammatory infiltrate and the fibrous scar11, since there was no difference between the LA group and the group that received SS, an apparently inert solution.
The application of long-lasting LA in the incision for postoperative analgesia has been associated with reduced cytokine-induced alterations, and in addition, it minimizes hyperalgesia14. Although some studies suggest that these drugs also have anti-inflammatory properties15, our results demonstrate that bupivacaine and ropivacaine, particularly the latter, are associated with larger inflammatory infiltrate. Furthermore, bupivacaine was associated with an area of infiltrating significantly larger than its levorotatory isomer, levobupivacaine.
A study using rats to evaluate inflammatory and cicatricial processes of the wound after long-lasting LA infiltration, assessing histology and tensile strength on the third and the fourteenth days, observed that, on the third day, there was a significant increase of macrophages in the group receiving bupivacaine. It was also observed that the collagen concentration was increased in the animals infiltrated with bupivacaine compared with ropivacaine and SS. There was no difference in the scar inflammatory response, presence of collagen, and tensile strength on the fourteenth day. The authors concluded that the alterations caused by LA infiltration do not extend beyond the third day, and therefore, do not impair the wound healing process in rats5. Our results contradict this supposition.
Another study on the effects of LA infiltration on the eighth day of wound healing in rats demonstrated that both bupivacaine and lidocaine reduced collagen production and resistance to scar rupture, causing significant edema, vascularization, and inflammation when compared with the controls4. Similarly, in the present study, we observed that bupivacaine was associated with the largest area of inflammatory infiltrate. Concerning the fibrous scar, bupivacaine seems not to interfere in the fibrous scar. On the other hand, its levorotatory isomer, levobupivacaine, and ropivacaine were associated with larger fibrous scar areas.
A study evaluating the effect of lidocaine and bupivacaine on wound healing in rats suggested that although these anesthetics influenced local inflammation and proteolytic factors, no effect on wound healing was observed16. These findings partially agree with ours.
Another study, using a methodology similar to ours, compared the amount of collagen and the number of mast cells through morphometry in rats after injection of lidocaine with epinephrine or with buffer. The authors concluded that lidocaine interferes with collagen and reduces the initial amount of mast cells in the surgical wound17.
Two experimental studies in rats associating levobupivacaine infiltration with ibuprofen and norepinephrine showed greater regeneration of the dermis and epidermis, granulation tissue and angiogenesis than in the control group, suggesting an increase in the regenerative/healing process6 and increased angiogenesis and tensile strength of the scar7. However, unlike our study, levobupivacaine was associated with a non-hormonal anti-inflammatory and a vasopressor, which could interfere with healing.
In rabbits with infiltration of lidocaine and bupivacaine, other authors found no histopathological difference when these LAs were compared with saline solution and suggested that these LAs do not affect healing18.
LAs act by directly inhibiting the nociceptive fibers of the skin. These fibers, together with the melanocytes, neuropeptides, and interleukins, are part of the cutaneous neuroendocrine system that, among other functions, modulate surgical healing, especially inflammation. LAs block neural impulses temporarily by inhibiting the neuroendocrine response to wound healing stimuli and related signaling, interfering negatively in wound healing. One of the neuropeptides that plays an essential role in the neuroendocrine system is the substance P, which controls mast cells’ degranulation and the release of inflammatory proteins in the surgical site. These cells act on wound healing by promoting inflammatory response, angiogenesis, and resorption of the extracellular matrix. In addition, they regulate growth factors and interleukins, essential for inflammation and the proliferative phase of wound healing19,20.
Amongst the different wound healing proteins, collagen correlates more closely with scar tissue strength. The amount of collagen in the scar site depends on the cicatricial process and may undergo LA interference due to inhibition during the neurogenic inflammatory phase21.
How SS 0,9% infiltration was associated with a larger area of inflammatory infiltrate and fibrous scar, we question whether, in addition to the action of LA or the SS 0.9% in the scar site, the mechanical effect (distension) caused by the infiltration interferes with the wound healing, possibility already described11.
When comparing bupivacaine with its isomer, levobupivacaine, the former was associated with the largest infiltrate area, and the last was associated with the largest fibrous scar areas and smallest area of infiltrate. Thus, this modification in the bupivacaine molecule could be responsible for the lower inflammation, and larger fibrous scar observed in the wound infiltrated with levobupivacaine.
CONCLUSION
The present study results indicate that the volume applied, or the infiltration trauma may have been responsible for the larger area de infiltrate and fibrous scar, not the LA itself. Complementary studies, including studies in humans, evaluating the equipotency between the LA and variation in the volume applied, are necessary to better understand the associated mechanisms, particularly in the evaluation of the area of inflammatory infiltrate and fibrosis in different stages (days) of healing, the type of inflammatory infiltrate and the cytokines involved.
REFERENCES
1. Lee NH, Ryu K, Song T. Postoperative analgesic efficacy of continuous wound infusion with local anesthetics after laparoscopy (PAIN): a randomized, double-blind, placebo-controlled trial. Surg Endosc. 2020 Fev;35(2):562-8.
2. Velanovich V, Rider P, Deck K, Minkowitz HS, Leiman D, Jones N, et al. Safety and efficacy of bupivacaine HCL collagen-matrix implant (INL-001) in open inguinal hernia repair: results from two randomized controlled trials. Adv Ther. 2019 Jan;36(1):200-16.
3. Brower MC, Johnson ME. Adverse effects of local anesthetic infiltration on wound healing. Reg Anesth Pain Med. 2003 Mai/Jun;28(3):233-40.
4. Hancı V, Hakimoğlu S, Özaçmak H, Bektas S, Özaçmak HS, Özadamar SO, et al. Comparison of the effects of bupivacaine, lidocaine, and tramadol infiltration on wound healing in rats. Rev Bras Anestesiol. 2012;62(6):799-810.
5. Abrão J, Fernandes CR, White PF, Shimano AC, okubo R, Lima BP, et al. Effect of local anaesthetic infiltration with bupivacaine and ropivacaine on wound healing: a placebo-controlled study. Int Wound J. 2014 Ago;11(4):379-85.
6. Zongwen G, Feng C, Xuemei C, Wang D, Li X, Li T. Local infiltration of the surgical wounds with levobupivacaine, dexibuprofen, and norepinephrine to reduce postoperative pain: a randomized, vehicle- controlled, and preclinical study. Biomed Pharmocother. 2017 Ago;92:459-67.
7. Korat PS, Kapupara PP. Local infiltration of the surgical wound with levobupivacaine, ibuprofen, and epinephrine in postoperative pain: an experimental study. Biomed Pharmacother. 2017 Dez;96:104-11.
8. Sakellaris G, Petrakis I, Makatounaki K, Arbiros I, Karkavitsas N, Charissis G. Effects of ropivacaine infiltration on cortisol and prolactin responses to postoperative pain after inguinal hernioraphy in children. J Pediatr Surg. 2004 Set;39(9):1400-3.
9. Paladini G, Di Carlo S, Musella G, Petrucci E, Scimia P, Ambrosoli A, et al. Continuous wound infiltration of local anesthetics in postoperative pain management: safety, efficacy and current perspectives. J Pain Res. 2020;31:285-94.
10. Joshi GP, Machi A. Surgical site infiltration: a neuroanatomical approach. Best Pract Res Clin Anaesthesiol. 2019 Set;33(3):317-24.
11. Pereira FEL. Reparo de lesões. In: Brasileiro Filho G, ed. Bogliolo patologia. 9ª ed. Rio de Janeiro: Guanabara Koogan; 2018. p. 181-93.
12. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003 Jul;83(3):835-70.
13. Vidmar J, Chingwaru C, Chingwaru W. Mammalian cell models to advance our understanding of wound healing: a review. J Surg Res. 2017 Abr;210:269-80.
14. Gordon SM, Brahim JS, Dubner R, McCullagh LM, Sang C, Dionne RA. Attenuation of pain in a randomized trial by suppression of peripheral nociceptive activity in the immediate postoperative period. Anesth Analg. 2002 Nov;95(5):1351-7.
15. Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand. 2006 Mar;50(3):265-82.
16. Waite A, Gilliver SC, Masterson GR, Hardman MJ, Ashcroft GS. Clinically relevant doses of lidocaine and bupivacaine do not impair cutaneous wound healing in mice. Br J Anaesth. 2010 Jun;104(6):768-73.
17. Rodrigues FV, Hochman B, Wood VT, Simões MJ, Juliano Y. Effects of lidocaine with epinephrine or with buffer on wound healing in rat skin. Wound Repair Regen. 2011;19(2):223-8.
18. Vasseur PB, Paul HA, Dybdal N, Crumley L. Effects of local anesthetics on healing of abdominal wounds in rabbits. Am J Vet Res. 1984 Nov;45(11):2385-8.
19. Robson MC. The role of growth factors in the healing of chronic wounds. Wound Repair Regen. 1997 Jan/Mar;5(1):12-7.
20. Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001 Fev;33(1):7-21.
21. Steinhoff M, Ständer S, Seeliger S, Ansel JC, Shmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003 Nov;139(11):1479-88.
1. Federal University of Triângulo Mineiro,
Uberaba, MG, Brazil.
2. University of Uberaba, Uberaba, MG,
Brazil.
MSC Analysis and/or data interpretation, Conceptualization, Final manuscript approval.
ECSA Analysis and/or data interpretation, Final manuscript approval, Methodology, Writing - Original Draft Preparation.
LAMS Conceptualization, Investigation, Methodology, Supervision.
GCO Analysis and/or data interpretation, Methodology, Writing - Original Draft Preparation.
ACG Conceptualization, Methodology, Writing - Original Draft Preparation.
BJM Conceptualization, Methodology, Writing - Original Draft Preparation.
RME Analysis and/or data interpretation, Conception and design study, Conceptualization, Final manuscript approval, Methodology, Supervision.
Corresponding author: Renata Margarida Etchebehere, RuaGetúlio Guaritá 140, Bairro Abadia, Uberaba, MG, Brasil, Zip Code 38025-440, E-mail: renata.etchebehere@uftm.edu.br
Article received: February 17, 2021.
Article accepted: April 19, 2021.
Conflicts of interest: none.
Institution: Hospital de Clínicas, Federal University of the Triângulo Mineiro, Uberaba, MG, Brasil..






 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter