

Case Report - Year 2021 - Volume 36 -
Extensive late skin lesion due to fluoroscopy ionizing radiation exposure: a case report
Extensa lesão cutânea tardia por exposição à radiação ionizante de fluoroscopia: relato de caso
ABSTRACT
The radiation-induced skin reaction (RCIR) is usually characterized by edema, hyperemia, fibrosis, ulceration, pain and itching on the skin. It is known that radiation disrupts the normal process of cell division and regeneration, resulting in damage that may involve impairment in the function of endothelial cells, inflammation and even cell death. The recovery of tissue damage by radiation depends on multiple factors related to the procedure performed and intrinsic to the patient. We present an atypical case of RCIR, whose lesions presented very unpredictable behavior and difficult clinical management. In addition, it is emphasized the importance of surgical intervention in this case, fundamental for the patient's proper treatment.
Keywords: Plastic surgery; Ionizing radiation; Necrosis; Fluoroscopy; Surgical flaps.
RESUMO
A reação cutânea induzida por radiação (RCIR) é geralmente caracterizada por edema, hiperemia, fibrose, ulceração, dor e prurido na pele. Sabe-se que a radiação interrompe o processo normal de divisão e regeneração celular, resultando em dano que pode envolver prejuízo na função das células endoteliais, inflamação e até morte celular. A recuperação do dano tecidual pela radiação depende de múltiplos fatores relativos ao procedimento realizado e também intrínsecos ao paciente. Apresentamos caso atípico de RCIR, cujas lesões apresentaram comportamento muito imprevisível e de difícil manejo clínico. Além disso, ressalta-se a importância da intervenção cirúrgica neste caso, fundamental para o tratamento adequado do paciente.
Palavras-chave: Cirurgia plástica; Radiação ionizante; Necrose; Fluoroscopia; Retalhos cirúrgicos.
INTRODUCTION
One of the most frequent adverse events of irradiation is skin reactions, occurring in up to 95% of patients. Its evolution depends on both characteristics of the treatment itself and risk factors intrinsic to the patient1.
The pathogenesis of radiodermatitis involves direct radiation injury and subsequent inflammatory response, affecting cellular elements in the skin. The energy of ionizing radiation produces immediate tissue damage by producing secondary electrons and reactive oxygen species. Each subsequent fraction of radiation generates greater recruitment of inflammatory cells, and the damage to the dermis disrupts the normal skin regeneration2,3. Thus, radiation-damaged skin has low healing power.
Compared with other fluoroscopically guided interventions, interventional cardiology procedures are associated with high doses of radiation directed to the skin, thus generating overdosing in prolonged fluoroscopies 4.
The effects of radiation on the skin can be classified as acute in the first six months and late after this period. Acute effects predominantly occur in tissues with a high level of mitotic activity and usually disappear within four weeks. Late effects, on the other hand, are secondary to radiation-induced vascular impairment and stromal fibrosis. In addition, hyper/hypopigmentation of the skin, fibrosis, telangiectasias and sebaceous and sweat gland dysfunction may occur5.
In the present article, we report an atypical case of radiodermatitis, aiming to discuss the approach of irradiated areas and their restoration difficulties as a result of radiation.
CASE REPORT
Caucasian male patient, 61 years old, hypertensive, dyslipidemic, obese, diabetic, hypothyroidism patient and smoker. History of disc herniation, two previous AMI (acute myocardial infarction). Currently in use of AAS, clopidogrel, enalapril, selozok, levothyroxine and atorvastatin. He was admitted to the plastic surgery service of the Hospital Monte Sinai de Juiz de Fora/MG for thoracic reconstruction due to extensive injury to the right thoracic back by fluoroscopic radiation.
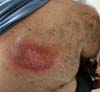
Submitted to angioplasty in June 2018, four stents were placed. Then, in August, he noticed the appearance of flushing and heat on the right-back, a topography that was coincident with the fluoroscope of hemodynamic procedures (Figure 1).
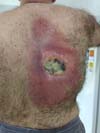
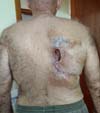
In September, he progressed to an ulcerated lesion of yellow-green color, measuring 12x8cm, located in the area of the radioscopy plate for catheterization (Figure 2). Again, there was a progressive increase in size, reaching deep planes.
Also, in September, a biopsy was performed, which showed dermal fibrosclerosis with reactive fibroblasts, steatonecrosis, tissue necrosis with abscess formation, and absence of malignancy in the cut-off planes examined: alterations compatible with radiotherapy effect.
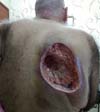
At this moment, debridement was performed, with primary closure of the lesion, but without success. At the end of September 2018 (Figure 3), he presented extensive necrosis and dehiscence of the scar. The lesion was left open, being oriented daily dressings and healing by second intention.
On February 26, 2019, showing significant improvement of the wound, he underwent excision of remaining actinic lesions and a new biopsy without complications. The histopathological report showed ulceration, hyper radioactive epidermis with apoptotic bodies, dermis with thick collagen bands, reactive fibroblasts, intense reactivity of the glandular epithelium, absence of malignancy, alterations compatible with the effect of radiotherapy, exceeding the resection margins (radiodermatitis necrosis). Again, healing was instructed by the second intention.
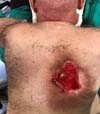
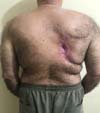
Due to the delay in wound healing, on October 22, 2019, he underwent reconstructive surgery with a detachment of a dermofat flap to cover the lesion (Figure 4). He evolved satisfactorily, being discharged on the third postoperative day (POD) for outpatient follow-up, after total integration of the surgical wound.
However, he presented a small dehiscence one month after the operation, showing her high healing deficit. Closing by the second intention was chosen on November 25, 2019 (Figure 5). The wound presented great difficulty in its healing process, with complete epithelialization of the surgical wound being detected only on April 7, 2020 (Figure 6).
DISCUSSION
RCIR are dose-dependent effects of ionizing radiation and usually occur when radiation dose limits are exceeded6.
Previous studies indicate that prolonged procedure times, multiple cumulative procedures, total occlusion of the right coronary artery, obesity, hypothyroidism, and diabetes are risk factors for RCIR7. In addition, the actual radiation dose required to cause deterministic skin injury is specific to each patient. It may vary widely based on individual biological variation, radiation sensitivity and the presence or absence of certain coexisting conditions8,9.
To date, there is no strong evidence to support the superiority of any specific preventive or therapeutic intervention in the treatment of RCIR. Therefore, a careful assessment of risk factors related to the development of skin toxicity remains a priority10.
It is important to highlight the great fragility of the irradiated tissue, which remains even after the apparent epithelialization of the wound from the RCIR. In our case, the patient underwent a small biopsy in a well epithelialized wound, but this traumatic stimulus was sufficient to aggravate a critical area again. Thus, irradiated tissue can remain intact for decades. However, any form of stress or tissue injury can generate a chronic wound exposed to noble structures. The treatment of these wounds usually requires extensive debridement of the necrotic skin, soft tissue, and affected bones, resulting in a complex wound, often with exposure to deep planes.
Because of the long delay in the second intention repair process, we chose to cover it with a dermofat flap, which is in line with what is recommended in the literature. Muscle and dermofat flaps from regions not affected by radiation can be useful for coverage and reconstruction 11. In addition, we believe that making the flap was essential for the proper treatment of this patient, including for their psychological comfort, as they were already undergoing daily dressings for a long time.
In the case presented, the persistence in the evolution of the necrosis of tissues on the back occurred for a long period, even after surgical debridement, with the comorbidities presented by the patient hampering wound healing. Due to the persistence of tissue damage by radiation, our patient evolved to massive tissue loss, which could affect the entire thickness of the chest wall and even have pulmonary involvement or even death.
Thus, the intervention of reconstructive plastic surgery was essential to improve the healing quality of the wound through debridement and flap making. In addition, the continuous follow-up of the patient associated with the clinical control of his comorbidities were fundamental measures for the delimitation and control of the progression of tissue necrosis, which evolved to good healing.
CONCLUSION
Skin lesions from exposure to ionizing radiation are associated with multiple factors. The case shown is related to the type of procedure performed, fluoroscopy, and comorbidities (obesity, diabetes, and thyroid diseases).
Even though all care was taken to restore the integrity of the patient’s chest, we evidenced the perpetuation of the radiation injury, an unusual fact to be observed even after successive surgical approaches.
REFERENCES
1. Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017 Set;56(9):909-14.
2. Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014 Jan;14:53.
3. Singh M, Alavi A, Wong R, Akita S. Radiodermatitis: a review of our current understanding. Am J Clin Dermatol. 2016 Jun;17(3):277-92.
4. Vance AZ, Weinberg BD, Arbique GM, Guild JB, Anderson JA, Chason DP. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34(8):1513-5.
5. Pruitt LG, Rogers W, Byarlay JA, Google PB. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016 Dez;43(12):1091-5.
6. Guesnier-Dopagne M, Boyer L, Pereira B, Guersen J, Motreff P, D'Incan M. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019 Mai;30(5):692-8.e13.
7. Weiss DJ, Pipinos II, Longo GM, Lynch TG, Rutar FJ, Johanning JM. Direct and indirect measurement of patient radiation exposure during endovascular aortic aneurysm repair. Ann Vasc Surg. 2008 Nov;22(6):723-9.
8. Kirkwood ML, Arbique GM, Guild JB, Timaran C, Valentine RJ. Radiation-induced skin injury after complex endovascular procedures. J Vasc Surg. 2014 Set;60(3):742-8.
9. Aragüés IH, Pérez AP, Fernández RS. Inflammatory skin conditions associated with radiotherapy. Actas Dermosifiliogr. 2017 Abr;108(3):209-20.
10. Iacovelli NA, Galaverni M, Cavallo A, Naimo S, Facchinetti N, Iotti C, et al. Prevention and treatment of radiation-induced acute dermatitis in head and neck cancer patients: a systematic review. Future Oncol. 2018 Fev;14(3):291-305.
11. Strojan P, Hutcheson KA, Eisbruch A, Beitler JJ, Langendijk JA, Lee AWM, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017 Sep;59:79-92.
1. Therezinha de Jesus Maternity Hospital, Juiz de Fora, MG, Brazil.
2. Monte Sinai Hospital, Juiz de Fora, MG, Brazil.
3. Faculty of Medical Sciences and Health of Juiz de Fora, Faculty of Medicine, Juiz
de Fora, MG, Brazil.
IPF Data Curation, Final manuscript approval, Writing - Original Draft Preparation, Writing - Review & Editing.
TGC Analysis and/or data interpretation, Final manuscript approval, Supervision, Writing - Review & Editing.
MTS Data Curation, Final manuscript approval, Writing - Review & Editing.
LFA Data Curation, Writing - Original Draft Preparation, Writing - Review & Editing.
Corresponding author: Iarley Peron Faria Rua Aníbal Maurício de Oliveira , nº 50, Centro, São João do Oriente, MG, Brazil Zip Code 35146-000 E-mail: iarleyperon@gmail.com / casalithais@hotmail.com
Article received: May 22, 2020.
Article accepted: April 23, 2021.
Conflicts of interest: none.









 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter