

Case Report - Year 2021 - Volume 36 -
Successful treatment of pyoderma gangrenosum after reducing mammoplasty: a case report
Tratamento exitoso de pioderma gangrenoso após mamoplastia redutora: relato de caso
ABSTRACT
Introduction: Pyoderma gangrenosum is a rare inflammatory disease characterized by the presence of ulcerated lesions. The etiology is unknown but apparently relates to immunological factors. The authors report a case of pyoderma gangrenosum in the postoperative period of a reduction mammoplasty, treatment, and the importance of early diagnosis to obtain a good aesthetic result.
Methods: This is an 18-year-old patient who developed pyoderma gangrenosum after a reduction mammoplasty. The initial symptoms were the formation of violet blisters and ulcerated lesions in both breasts. The disease was suspected, and the patient was promptly treated with prednisolone. Cultures, biopsies and blood tests were performed. Hyperbaric therapy and nutritional support were performed. Daily dressings with epidermal growth factors were performed. After 60 days of follow-up, it was possible to achieve adequate wound healing and good aesthetic results.
Results: Early diagnosis associated with systemic immunosuppressive therapy and rigorous local care were fundamental for disease stabilization. The association with hyperbaric therapy and nutritional support contributed to the lesion's epithelialization and the clinical picture control. The approximation of the wound edges with subdermal suture and the subsequent intradermal suture was possible due to the recovery of the dermis and stabilization of the disease.
Conclusion: Early diagnosis of pyoderma gangrenosum is essential to achieve stabilization of the disease. It is possible to achieve good aesthetic results based on four elements: early systemic corticosteroids, daily local dressings with epidermal growth factor, nutritional support, and hyperbaric therapy.
Keywords: Pyoderma gangrenosum; Breast disease; Mammoplasty; Autoimmune diseases; Skin disease; Mama.
RESUMO
Introdução: O pioderma gangrenoso é uma doença inflamatória rara caracterizada pela presença de lesões ulceradas. A etiologia é desconhecida, mas aparentemente relaciona-se com fatores imunológicos. Os autores relatam um caso de pioderma gangrenoso no pós-operatório de uma mamoplastia redutora, o tratamento e a importância do diagnóstico precoce para obtenção de um bom resultado estético.
Métodos: Trata-se de uma paciente de 18 anos que desenvolveu pioderma gangrenoso após uma mamoplastia redutora. Os sintomas iniciais foram a formação de bolhas de coloração violácea e lesões ulceradas em ambas mamas. Suspeitou-se a doença e a paciente foi tratada prontamente com prednisolona. Foram realizadas culturas, biópsias e exames sanguíneos. Realizou-se terapia hiperbárica e suporte nutricional. Foram realizados curativos diários com fator de crescimento epidérmico. Após 60 dias de acompanhamento foi possível conseguir uma cicatrização adequada da feridas e bons resultados estéticos.
Resultados: O diagnóstico precoce associado a uma terapia sistêmica imunossupressora e cuidados locais rigorosos foram fundamentais para a estabilização da doença. A associação com terapia hiperbárica e o suporte nutricional contribuíram para a epitelização das lesões e o controle do quadro clínico. A aproximação das bordas da ferida com sutura subdérmica e posterior sutura intradérmica foram possíveis devido à recuperação da derme e estabilização da doença.
Conclusão: O diagnóstico precoce de pioderma gangrenoso é fundamental para conseguir a estabilização da doença. É possível atingir bons resultados estéticos baseados em 4 elementos: corticoide sistêmico precoce, curativos locais diários com fator de crescimento epidérmico, suporte nutricional e terapia hiperbárica.
Palavras-chave: Pioderma gangrenoso; Doença da mama; Mamoplastia; Doenças autoimunes; Doença da pele; Mama.
introduction
Pyoderma gangrenosum (PG) is an inflammatory skin disease characterized by the presence of ulcerative lesions with violaceous and indeterminate borders1. Usually, the clinical picture is rapidly progressive with the extension of necrotic areas in a serpiginous configuration2. The etiology is unknown to this day but is believed to involve neutrophilic dysfunction and immunological factors. It is often associated with inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis, malignancies, arthritis, and hematological diseases. In many cases, the clinical presentation can simulate a surgical wound infection3.
Brocq first described this pathology in 19163 and later named and better characterized by Brunsting in 19304,5. PG was so called by the author, believing that the pathology was gangrene caused by streptococcal infection. The first case described in breast surgery was in 1988, by Rand6, in a patient undergoing a reduction mammoplasty.
Pyoderma gangrenosum has an estimated incidence of 3 to 10 cases per million people/year7,8, with reports in the literature involving single cases or small series of patients. National data from a retrospective analysis showed that, in Brazil, this index is 0.38 cases per 10,000 visits9, with women being the most affected, between the second and fifth decade of life10.
Histopathological findings include sterile dermal neutrophilia and lymphocytic vasculitis but are nonspecific, both in optical microscopy and immunohistochemistry, with no specific serological markers for closing a laboratory diagnosis; thus, the diagnosis is clinical by exclusion11,12. In addition, PG is associated with some autoimmune diseases, such as ulcerative colitis and rheumatoid arthritis13.
Pathergy is a phenomenon observed in patients diagnosed with PG and suggests an abnormal response to trauma. This characteristic makes the treatment of this pathology more difficult compared to other ulcerative diseases3,14. Until now, the literature suggests treatments based on corticosteroids, where despite achieving disease control, we evidence irreparable skin lesions with an unwanted final result15.
CASE REPORT
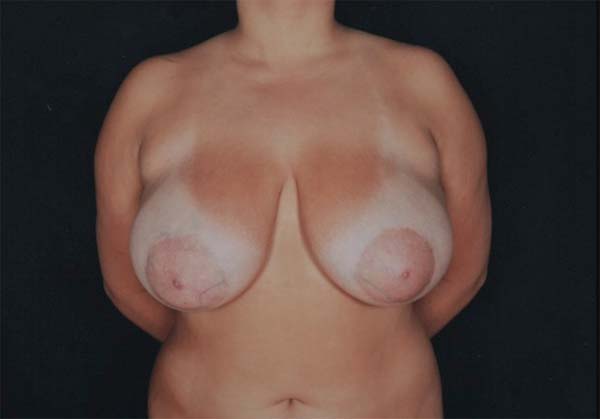
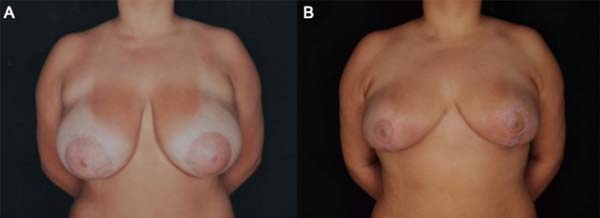
A 17-year-old female patient without any pathological or surgical history arrived at the plastic surgery outpatient clinic complaining of breast hypertrophy that generated back pain and social discomfort (Figure 1). The physical examination showed macromastia and grade I breast ptosis.
She underwent reduction mammoplasty with lower pedicle, surgery performed without complications, being discharged after 24 hours of hospitalization in good general condition. Antibiotic prophylaxis with cefazolin was performed during surgery, and subsequently, 500mg cephalexin of 6/6 hours for five days was prescribed.
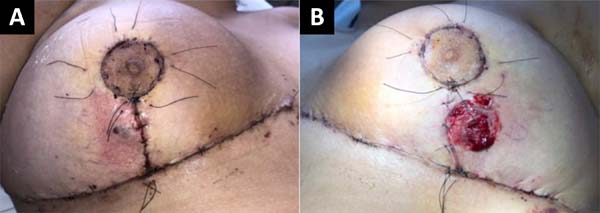
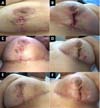
In the outpatient control on the seventh postoperative day (POD), a vesicle with hematic content with perilesional erythema appeared in the lower outer quadrant of the right lateral breast. In the left breast, the patient had a bleeding ulcer of approximately 4.5 cm in diameter in the lower external and lateral quadrant (Figure 2).
A diagnosis of pyoderma gangrenosum was suspected, for which a biopsy of the wound was performed with collection and culture material. Subsequently, a dressing was performed with non-adherent gauze and blood was collected for complete blood count, C-reactive protein and erythrocyte sedimentation rate. Then, for ten days, oral treatment with 40mg/day prednisolone plus amoxicillin with clavulanate 875+125mg 12/12h.
Laboratory test results showed 11,270 leukocytes/µL with 9,650 neutrophils/µL (85%), 94mm ESR and PCR 2.28mm/dL. The histopathological report of the lesion reported: skin fragment on the edge of an ulcerated lesion showing acute and chronic inflammatory infiltrate in the dermis, and subcutaneous cell tissue with fibrinoid necrosis of the wall of small vessels related to the ulcer area. Absence of microorganisms to special stains by PAS and Ziehl-Neelsen. Consider pyoderma gangrenosum among diagnoses.
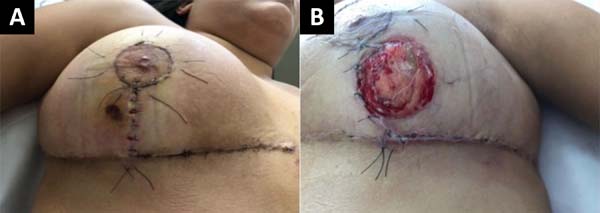
At 18° DPO, ulcerated lesions still appeared to have a slow progression, showing a slight increase in the size of the left breast ulcer that even compromised the vertical scar. Daily sessions of hyperbaric therapy with a duration of 90 minutes associated with nutritional support with protein supplement: Cubitan® (Vital Products Importação e Exportação Ltda., Niterói, Rio de Janeiro, Brazil) 1 vial per day and glutamine 10 grams per day (Figure 3).
From the 20th DPO, daily dressings were started with epidermal growth factor at 3%, rosehip oil 5% and allantoin 1% covered with non-adherent gauze.
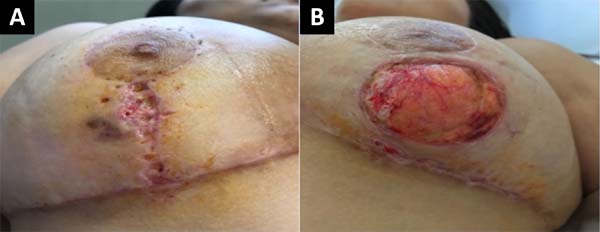
On the 25th POD, it was possible to evidence a favorable evolution of the wounds due to an arrest in their extension associated with an apparent attempt to epithelialize the wound edges (Figure 4).
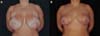
A subdermal suture was performed on the 33rd POD with nylon 3.0 to approximate the edges of the lesion without showing any pathergic reaction due to the manipulation of the wound. One week later, the skin was sutured with a 5.0 nylon suture associated with simple stitches. According to the healing of the patient’s skin, the stitches were gradually removed (Figure 5).

RESULT
The patient, in this case, 60 days after surgery, evolved with good healing of the extensive lesions, and the result of mammoplasty without sequelae from PG was preserved (Figure 6). When contacted by the team, the patient was satisfied with the result of mammoplasty, with three years of postoperative segment from the surgical procedure.
DISCUSSION
Post-surgical pyoderma gangrenosum is a skin disease developed soon after surgery, mimetizing a surgical wound infection that does not respond to antibiotic treatment. More and more cases are reported in the literature so that it represents a pathology that every plastic surgeon must know to perform an adequate differential diagnosis16,17. In a recent review in the national literature, in the Brazilian Journal of Plastic Surgery, six articles have been published since 201518-23 and 10 since 20069,14,24,25, being fundamental the suspicion of PG from pathognomonic lesions in surgeries with catastrophic and recent evolutions.
Early diagnosis is essential to prevent the progression of ulcerative lesions and to carry out appropriate treatment. In the literature, the treatment indicated in most cases and based on corticosteroids associated or not with cyclosporine1,26. In addition, the use of methotrexate, cyclophosphamide, azathioprine, and immunoglobulin has been described in patients who have not responded to steroid treatment15,27. In the patient in the case described, a favorable outcome of this dreaded pathology, which has a sudden and aggressive onset, was obtained due to clinical suspicion and early treatment.
Due to the characteristic pathergy of this disease, the tendency is not to perform surgical debridement and manipulate the wounds. Some authors describe the performance of grafts and flaps once the disease is controlled to close the bloody area13. However, most of the aesthetic results found in the literature are poor. The patient in question had no history of previous injury, either from the PG or the pathergy phenomenon. No report of previous surgery or major trauma. Having good evolution with the interventions of approximation suture and associated with suturing the wound in planes, without debridement of the region.
In the patient of the case, good aesthetic results were obtained due to a diagnosis and early corticosteroid therapy that limited the extent of the ulcerated region. Later, with nutritional support, hyperbaric therapy and daily dressings controlled the disease, promoting wound epithelialization. Finally, it was possible to perform a wound closure by stages, leaving minimal scars on the breasts.
CONCLUSION
Post-surgical pyoderma gangrenosum is a disease that must be known by the plastic surgeon to be properly treated and ensure good aesthetic results for patients. Diagnostic suspicion is made when the patient presents ulcerative lesions in the early postoperative period, usually multiple, bilateral, and sparing the areola-papillary complex.
It is possible to achieve control of the disease with the stabilization of the disease with corticosteroid therapy associated with nutritional support, hyperbaric therapy, and daily dressings.
Once the disease is controlled, it is possible to manipulate the affected areas by achieving a late closure of the ulcers and adequate healing.
REFERENCES
1. Larcher L, Schwaiger K, Eisendle K, et al. Aesthetic breast augmentation mastopexy followed by postsurgical pyoderma gangrenosum (PSPG): clinic, treatment, and review of the literature. Aesth Plast Surg. 2015;39:506-13.
2. Abtahi-naeini B, Bagheri F, Pourazizi M, Forozeshfard M, Saffaei A. Unusual cause of breast wound: postoperative pyoderma gangrenosum. Int Wound J. 2017;14:285-7.
3. Brocq L. Nouvelle contribution a l’etude du phagedenisme geometrique. Ann Dermatol Syph 1916; 6: 1-39.
4. .Brunsting LA, Goeckerman WH, O´Leary PA. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occurring in adults. Arch Dermatol Syph 1930; 22: 655-80.
5. Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J. 2012;3:7-13.
6. Rand RP, Brown GL, Bostwick J. Pyoderma gangrenosum and progressive cutaneous ulceration. Ann Plast Surg. 1988;20:280Y284.
7. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191- 211.
8. Bolognia JL, Jorizzo JL, Rapini RP. Pyoderma gangrenosum. Dermatology.2003;1:415-8.
9. Meyer TN. Pioderma gangrenoso: grave e mal conhecida complicação da cicatrização. Rev Bras Cir Plást. 2006;21(2):120-4.
10. Suárez-Pérez JA, Herrera-Acosta E, López-Navarro N, Vilchez-Márquez F, Prieto JD, Bosch RJ, et al. Pioderma gangrenoso: presentación de 15 casos y revisión de la literatura. Actas Dermosifiliogr. 2012;103(2):120-6.
11. Souza CS, Chiossi MPV, Takada MH, Foss NT, Roselino AMF. Pioderma gangrenoso: casuística e revisão de aspectos clínico-laboratoriais e terapêuticos. An Bras Dermatol. 1999;74(5):465-72.
12. Konopha CL, Padulla GA, Ortiz MP, Beck AK, Bitencourt MR, Dalcin DC. Pioderma gangrenoso: um artigo de revisão. J Vasc Bras. 2013;12(1):25-33.
13. Tuffaha S, Sarhane K, Mundinger G, Pyoderma gangrenosum after breast surgery: diagnostic pearls and treatment recommendations based on a systematic literature review. Ann Plast Surg. 2016;77:e39Ye44.
14. Coltro PS, Valler CS, Almeida PCC, Gomez DS, Ferreira MC. O Papel da Patergia no Pioderma Gangrenoso em Áreas Doadoras de Enxertos Cutâneos: Relato de Caso. Rev. Bras. Cir. Plást.2006;21(4):231-235.
15. Grillo MA, Cavalheiro TT, Mulazani MS, et al. Postsurgical pyoderma gangrenosum complicating reduction mammaplasty. Aesthetic Plast Surg. 2012;36:1347-52.
16. Segaran A, Mohammad M, Sterling JC, et al. Pyoderma gangrenosum arising in a breast reduction scar: seven years post-procedure. J Plast Reconstr Aesthet Surg. 2013;66:e370-e2.
17. Duval A, Boissel N, Servant JM, et al. Pyoderma gangrenosum of the breast: a diagnosis not to be missed. J Plast Reconstr Aesthet Surg. 2011;64:e17-e20.
18. Sempértegui A, Vasquez ISV, Fernandes Neto ALRG, Santos LML, Alvarenga BCF, Ueda WJ, et al. Pioderma gangrenoso após quadrantectomia de mama com radioterapia intraoperatória: relato de caso. Rev Bras Cir Plást. 2015;30(1):138-42.
19. Rosseto M, Costa SC, Narváez PLV, Nakagawa CM, Costa GO. Pioderma gangrenoso em abdominoplastia: relato de caso. Rev Bras Cir Plást. 2015;30(4):654-7.
20. Oliveira FFG, Fernandes M, Giacoia AMN, Saldanha O, Menegazzo MR, Cação EG, et al. Pioderma gangrenoso: um desafio para o cirurgião plástico. Rev Bras Cir Plást. 2018;33(3):414-8.
21. Gaspar-Junior CJ, Gaspar CJ. Pioderma gangrenoso em pós-operatório de mamoplastia redutora: relato de caso. Rev Bras Cir Plást. 2018;33(4):590-4.
22. Yamaki IN, Boechat CJ, Rizzo RC, Amorim SS, Andrade GF, Reis AP. Pioderma gangrenoso após mamoplastia redutora: desafios diagnóstico e terapêutico. Rev Bras Cir Plást. 2019;34(4):567-70.
23. Rezende AV, Macedo JL, Rosa SC, Yamamoto HG, Fioravante TGR, Garcia JEF, et al. Pioderma gangrenoso em dorso de mão pós-trauma. Rev Bras Cir Plást. 2020;35(1):121-8.
24. Furtado JG, Furtado GB. Pioderma gangrenoso em mastoplastia e abdominoplastia. Rev Bras Cir Plást. 2010;25(4):725-7.
25. Soares JM, Rinaldi AE, Taffo E, Alves DG, Cutait RCC, Estrada EOD. Pioderma gangrenoso pós-mamoplastia redutora: relato de caso e discussão. Rev Bras Cir Plást. 2013;28(3):511-4.
26. Juhász ML, Maman DY, Levin JM, et al. Pyoderma gangrenosum of the breast in a patient with a history of silicone augmentation mastopexy and suction-assisted lipectomy of the trunk. JAAD Case Rep. 2015;1:329-32.
27. Poucke SV, Jorens PG, Peeters R, Jacobs W, Beeck BO, Lambert J, et al. Pyoderma gangrenosum: a challenging complication of bilateral mastopexy. Int Wound J. 2004;1:207-13.
1. Iguaçu University, Hospital Niterói D’Or, Plastic Surgery, Niterói, RJ, Brazil.
GHP Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Methodology, Project Administration, Supervision, Writing - Original Draft Preparation, Writing - Review & Editing.
FSMCF Analysis and/or data interpretation, Data Curation, Final manuscript approval, Methodology.
LAVG Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Methodology, Project Administration, Writing - Original Draft Preparation, Writing - Review & Editing.
Corresponding author: Gisela Hobson Pontes Avenida Epitácio Pessoa, 846, Ipanema, Rio de Janeiro, RJ, Brazil Zip Code 22410-090 E-mail: giselapontes@uol.com.br
Article received: April 05, 2020.
Article accepted: April 23, 2021.
Conflicts of interest: none.







 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter