

Original Article - Year 2021 - Volume 36 -
Analysis of the results of surgical treatment using the sural flap
Análise dos resultados do tratamento cirúrgico utilizando o retalho sural
ABSTRACT
Introduction: Complex fractures and extensive skin lesions are increasingly common due to high-energy traumas. An alternative for treating these lesions in the lower limbs is the use of the sural flap.
Methods: This was a retrospective, analytical-descriptive study of exploratory documental analysis of patients submitted to the sural flap in a trauma reference hospital in northern Santa Catarina, Brazil. Age, sex, laterality, cause, place, and size of the lesion, use of tunneling and skin grafting, complications and their risk factors, and the management of such complications were analyzed.
Results: The study sample consisted of 16 patients with a mean age of 44.4 years; 87.5% were male. The cause of the most prevalent lesion was trauma (75.0%), and the site of the lesion was more prevalent in the distal tibia (43.8%). In 50.0% of the cases, risk factors for complications were present, and patients with diabetes mellitus and smokers were five times more likely to present such complications. Partial necrosis had a prevalence of 25.0%, and in 18.8%, only debridement was performed, and 6.3% grafting was performed.
Conclusion: The sural flap is a good alternative for covering lower limbs lesions due to its good success rate, but it is not free of complications. Such complications are more prevalent in patients who have risk factors such as smoking and diabetes mellitus.
Keywords: Myocutaneous flap; Sural nerve; Necrosis; Smoking; Diabetes mellitus.
RESUMO
Introdução: Fraturas complexas e extensas lesões de pele estão cada vez mais comuns devido aos traumas de alta energia. Uma alternativa para o tratamento dessas lesões nos membros inferiores é a utilização do retalho sural.
Métodos: Esse foi um estudo retrospectivo, analítico-descritivo de análise exploratória documental de pacientes submetidos ao retalho sural em um hospital de referência em trauma do norte de Santa Catarina, Brasil. Foram analisados a idade, sexo, lateralidade, causa, local e tamanho da lesão, uso de tunelização e enxerto de pele, complicações e seus fatores de risco, além do manejo de tais complicações.
Resultados: A amostra do estudo foi composta por 16 pacientes, com média de idade de 44,4 anos, 87,5% eram do sexo masculino. A causa da lesão mais prevalente foi trauma (75,0%) e o local da lesão foi mais prevalente na tíbia distal (43,8%). Em 50,0% dos casos os fatores de risco para as complicações estavam presentes, sendo que pacientes com diabetes mellitus e tabagistas exibiram 5 vezes mais chances de apresentar tais complicações. Necrose parcial teve uma prevalência de 25,0%, sendo que em 18,8% foi realizado apenas debridamento e em 6,3% enxertia.
Conclusão: O retalho sural é uma boa alternativa para a cobertura de lesões dos membros inferiores devido ao bom índice de sucesso, mas não está livre de complicações. Tais complicações são mais prevalentes em pacientes que possuem fatores de risco como o tabagismo e diabetes mellitus.
Palavras-chave: Retalho miocutâneo; Nervo sural; Necrose; Tabagismo; Diabetes mellitus
INTRODUCTION
Due to the increase in high-energy trauma, complex fractures associated with extensive skin lesions have become more frequent1. Places such as the distal extremity of the leg, ankle, heel and foot are a reconstructive challenge. They are areas susceptible to trauma, plantar support, where chronic ulcers and pressure can occur, besides having scarce subcutaneous tissue and bony prominences2.
An alternative for the treatment of these areas in the lower limbs is the use of the sural flap, which was first described by Donski and Fogdestam (2015)6 Apud and later detailed by Masquelet et al. in 19924, who introduced the concept of a neurocutaneous island flap and described the sural neurocutaneous flap, known as the sural retrograde flow flap2-4.
The sural flap has many advantages such as being easy and fast to perform, high survival rates, fast learning curve1, preserves important arteries and muscles, in addition to great mobility, versatility and flexibility, thus presenting a good rotation arc3,5.
It is not recommended to perform a flap in a large area (>9x12cm) for the distal third of the leg due to the risk of poor perfusion and for regions where sensitivity would be of great importance due to the sacrifice of the sural flap. Contraindication is limited to the previous lesion of the vascular pedicle or the inferior perforating of the sural artery6. But it is also associated with congestion due to poor venous drainage, skin necrosis, unpleasant scars at the donor site and sensory disorders5,7. Such complications may be due to some risk factors such as age, diabetes mellitus, obesity, peripheral vascular disease and smoking2,5.
OBJECTIVE
Perform a retrospective analysis of the results obtained in the surgical treatment of lower limbs lesions using the sural flap.
METHODS
This was a retrospective, analytical-descriptive study of exploratory documental analysis, approved by the institution’s research ethics committee, which was conducted under number 29498220.3.0000.5362.
Medical records of patients submitted to surgical treatment using the sural flap were evaluated to cover lower limbs lesions from January 2015 to March 2020.
The variables studied in this study were age, gender, laterality, cause, place, and size of the lesion, use of tunneling and skin grafting, complications and their risk factors, in addition to the management of such complications.
All procedures were performed by the same hand surgeon and microsurgery team 7-8, and the surgical technique used was based on the description of Masquelet et al. (1992) 4, in which the patient in ventral or lateral decubitus was submitted to the anesthetic procedure in addition to prophylactic antibiotic therapy for 24 hours (cefazolin).
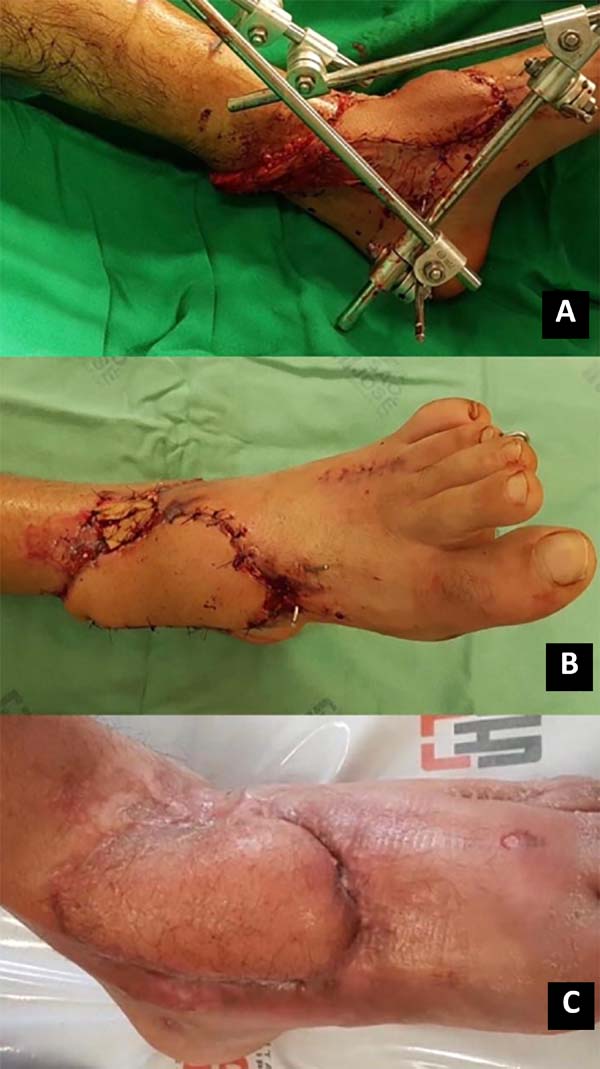
The pivot point of the flap was marked approximately 5 cm above the lateral malleolus; the surgical incision in the posterior part of the leg, between the muscle bellies of the gastrocnemius muscles, from the skin to the sural fascia, where the vasculonervous bundle is found. Dissection was performed from proximal to distal; the superficial sural nerve and artery, in addition to the saphenous vein, were proximally connected, so the flap that includes the fascia was elevated to the point of rotation.
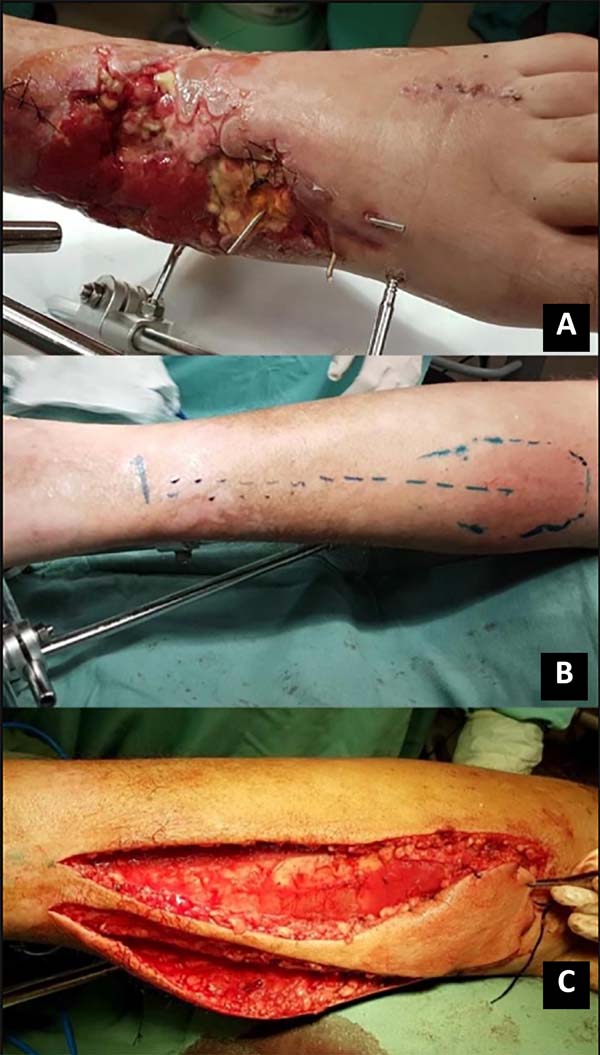
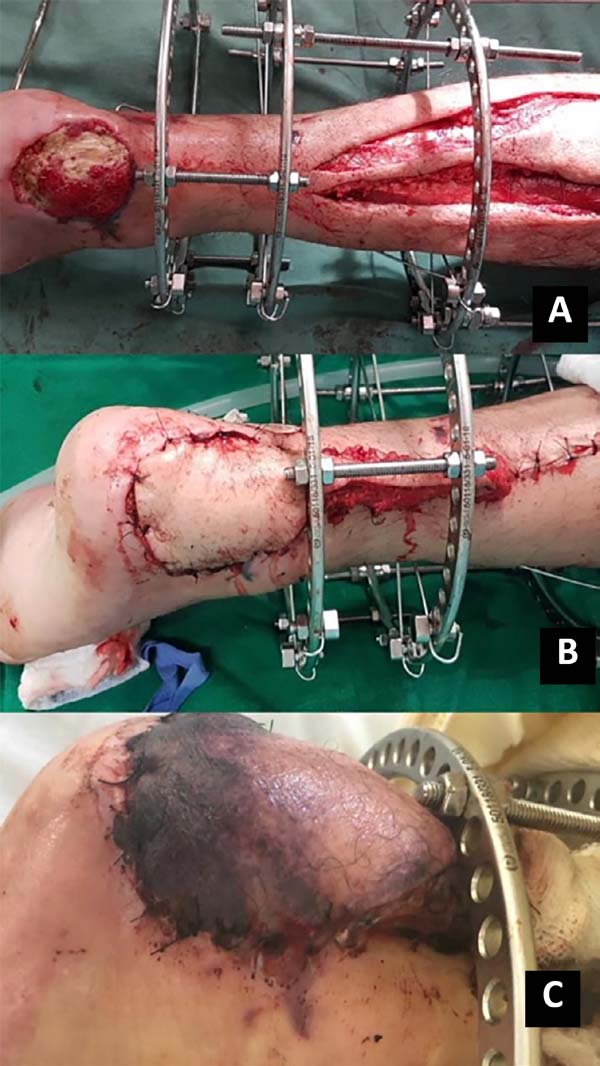
A subcutaneous tunnel may transport the flap, or the pedicle may be externalized with a skin syrup (having to undergo a second procedure for pedicle removal); flap rotation and suture only of the skin with nylon 3.0 in the receiving area was performed. Soon after, the perfusion of the flap was evaluated. If possible, the donor area is closed primarily (island less than 4cm); if necessary, the partial-thickness skin graft of the ipsilateral thigh is removed for coverage (Figures 1 and 2).
A sterile dressing was performed with gauze and orthopedic cotton, taking care not to compress the flap; the drain was removed within 48 hours after the surgical procedure. The flap was monitored 4 in 4 hours in the first 48 hours, and the patient was instructed not to use foods that could cause vasoconstriction, such as chocolate and coffee.
Data inferential analysis was performed using the IBM Statistical Package for the Social Sciences (SPSS) version 22.0 software.
Descriptive analysis was used to characterize the sample and lesion characteristics, using ± standard deviation (SD) for quantitative variables and absolute number (n) and percentage (%).
To compare the outcome: partial necrosis complication and the association with possible predictor variables, Poisson regression was used to ascertain the relative risk (RR). A p-value α =0.05 (p≤0.05) was adopted as the level for statistical significance.
RESULTS
The present study sample was composed of sixteen (n=16) patients who underwent surgical treatment of lower limbs lesions using the sural flap (Table 1). The mean age was 44.4±17.7 years, and the highest prevalence was male 87.5% (n=14) (Table 2).
| No. | Sex | Age (years) | Size (cm) | Cause | Local | Risk factors | Complications |
|---|---|---|---|---|---|---|---|
| 1 | M | 57 | 8x6 | Trauma | Calcaneus | SAH/Tab | No |
| 2 | M | 17 | 6X7 | Trauma | Distal tibia | Tab | No |
| 3 | F | 57 | 6X5 | Chronic ulcer | Distal tibia | SAH | No |
| 4 | M | 26 | 7X6 | Trauma | Calcaneus | No | No |
| 5 | M | 38 | 7X7 | Trauma | Back of the foot | No | No |
| 6 | M | 38 | 8x6 | Trauma | Distal tibia | No | Partial necrosis |
| 7 | M | 67 | 7X3 | Chronic ulcer | Calcaneus | SAH/Tab | Partial necrosis |
| 8 | M | 22 | 6X6 | Trauma | Distal tibia | No | No |
| 9 | M | 74 | 7X4 | Chronic ulcer | Calcaneus | SAH/PVD | No |
| 10 | M | 53 | 7X6 | Trauma | Calcaneus | DM/Tab | Partial necrosis |
| 11 | M | 36 | 11x7 | Trauma | Back of the foot | No | No |
| 12 | M | 56 | 12X6 | Trauma | Distal tibia | No | No |
| 13 | M | 60 | 8x6 | Trauma | Distal tibia | SAH | Partial necrosis |
| 14 | M | 20 | 14X7 | Trauma | Back of the foot | No | No |
| 15 | F | 55 | 5x3 | Trauma | Distal tibia | No | No |
| 16 | M | 34 | 15X10 | Chronic ulcer | Calcaneus | Tab | No |
The cause of the most prevalent lesion was trauma 75.0% (n=12), and the site of the lesion was more prevalent in the distal tibia 43.8% (n=7), with an average size of 52.9±33.4cm2. Tunneling of the pedicle was performed and grafting in 87.5% (n=14) of the lesions. Regarding risk factors, 50.0% (n=8) had no risk factors. On the possible complications during outpatient follow-up, partial necrosis had a prevalence of 25.0% (n=4). In 18.8% (n=3), only debridement was performed, and in 6.3% (n=1), partial skin grafting at the complication sites (Table 3).
| Characteristics of the lesion | n=16 |
|---|---|
| Cause of injury - n (%) | |
| Trauma | 12 (75.0) |
| Chronic ulcer | 4 (25.0) |
| Site of injury - n (%) | |
| Distal tibia | 7 (43.8) |
| Calcaneus | 6 (37.5) |
| Back of the foot | 3 (18.8) |
| Lesion size (cm 2) - mean ±SD | 52.9±33.4 |
| Lesion size (cm2) - median (IIQ) | 45 (31.5-66.25) |
| Pedicle tunneling - n (%) | |
| No | 14 (87.5) |
| Yes | 2 (12.5) |
| Grafting in the donor area - n (%) | |
| Yes | 14 (87.5) |
| No | 2 (12.5) |
| Risk factors - n (%) | |
| No | 8 (50.0) |
| Smoking | 2 (12.5) |
| SAH | 2 (12.5) |
| SAH and smoking | 2 (12.5) |
| SAH and PVD | 1 (6.3) |
| DM and smoking | 1 (6.3) |
| Complications - n (%) | |
| No | 12 (75.0) |
| Partial necrosis | 4 (25.0) |
| Management - n (%) | |
| Debridement | 3 (18.8) |
| Grafting | 1 (6.3) |
| Unspecified | 12 (75.0) |
The prevalence of partial necrosis outcome was 25.0% in the sample studied. It is observed in the univariate analysis between the outcome and the predictor variables, which have as risk factors before surgery, diabetes mellitus and being a smoker, is a risk factor 5 times greater of having as partial complication necrosis of the flap (RR: 5.00; CI 95%:1.82-13.76; p≤0.05) (Table 4).
| n (%) | Gross RR | CI 95% | p | |
|---|---|---|---|---|
| Sample characterization | ||||
| Age - ≥55 years | 8 (50.0) | 3.00 | 0.39-23.1 | 0.29 |
| Gender - Male | 14 (87.5)) | - | - | - |
| Characterization of the lesion | ||||
| Cause - Trauma | 12 (75.0) | 1.00 | 0.14-7.10 | 1.00 |
| Location - Calcaneus | 6 (37.5) | 1.67 | 0.33-8.93 | 0.55 |
| Location - Distal Tibia | 7 (43.8) | 1.29 | 0.24-6.99 | 0.77 |
| Location - Back of the foot | 3 (18.8) | - | - | - |
| Tunneling | 2 (12.5) | 2.33 | 0.42-12.9 | 0.33 |
| Risk factors - Yes | 8 (50.0) | 3.00 | 0.39-23.07 | 0.29 |
| Risk factors - SAH | 2 (12.5) | 2.33 | 0.42-12.9 | 0.33 |
| Risk Factors - Smoking | 2 (12.5) | - | - | - |
| Risk factors - SAH and smoking | 2 (12.5) | 2.33 | 0.42-12.9 | 0.33 |
| Risk factors - DM and smoking | 1 (6.3) | 5.00 | 1.82-13.76 | 0.01* |
| Risk factors - SAH and PVD | 1 (6.3) | - | - | - |
| Management - Debridement | 3 (75.0) | - | - | - |
| Management - Grafting | 1 (25.0) | - | - | - |
n = Sample number; SAH = Systemic arterial hypertension; CI = Confidence interval; RR = Relative risk; SAH = Systemic arterial hypertension; DM = Diabetes mellitus; PVD = Peripheral vascular disease; p: Statistical inference;
* There was a statistically significant difference (p≤0.05).
DISCUSSION
Based on the results obtained, the complications of the sural flap can be correlated concerning risk factors, besides evaluating the variation of the determinants of the evolution of the disease related to treatment.
In other studies, similar data were found in ours, in which young patients with a mean age of 44 years and males were the most prevalent, probably due to the cause of the injury being the vast majority due to trauma1,2,5,9.
It was observed the similarity of results, in other studies, that the distal third of the leg and calcaneus were the most prevalent sites of the lesions. This study also observed these results, being present in 7 cases in the distal third of the leg, followed by the calcaneus with 6 cases2,10.
According to Quirino and Viegas (2014)11, there was a need for partial skin grafting in the donor area in 11 of the 12 patients in their study, a result like ours, performed in 14 patients. We chose to tunnel in only 2 cases in our work, while Quirino and Viegas (2014)11 performed in 5 cases. For Vendramin (2012)10, the choice may vary according to the location and the presence of complications already observed in the intraoperative period, such as vascular compression of the pedicle; we used the same criteria for the decision of tunneling. In our study, it did not influence the final result of the flap, a fact also found in the work of Parrett et al. (2009)12.
We considered a good final result in the treatment using the sural flap because, in only 25%, there was partial necrosis and in no case the total necrosis of the flap (Figure 3). Such data are similar to those found in other studies such as that of Singh et al. (2017) 1, in which there was partial necrosis in 2 of the 15 patients studied. In contrast, Ciofu et al. (2017) 13 reported a complication rate of 30% in a population of high-risk patients, including patients with diabetes mellitus, peripheral vascular disease, and venous insufficiency. Vendramin (2012)10 obtained partial necrosis in 18.5% and total necrosis in 3.7% in the first phase of his work, in which the author had performed the surgeries with less experience with the technique. These results improved in the second phase, with more experience. There was partial necrosis in only 8.8% of the cases, being the surgeon’s experience a factor that can interfere in the outcome of the surgery and better results in future cases.
Diabetes mellitus, hypertension, smoking and peripheral vascular disease are risk factors for postoperative complications, and they were present in 50% (8 cases) of the patients in the study. That is a number greater than that found in the work of Severo et al. (2019) 5, in which risk factors were observed in 3 of the 24 medical records and in Garcia’s (2009)2 that was found in 6 of the 15 patients. Therefore, this may be a determining factor for lower rates of partial necrosis such as those of Severo et al. (2019) 5 with 8.3% and Garcia (2009)2 with only one case reported. This fact was proven with the study by Parrett et al. (2009) 12, who, comparing only the group of patients with diabetes, peripheral vascular disease and smoking, had complications in 78% of the cases, different from the group without comorbidities, with 16%.
When compared with other risk factors, this becomes even more evident, as demonstrated by Baumeister et al. (2003) 14 who presented complications in 11% of healthy patients, 33% in patients with coexisting diseases, such as hypertonia, obesity, coronary artery disease or paraplegia, and 60% in diabetics, with venous insufficiency and peripheral arterial disease probably caused by macro and microangiopathy caused by these diseases. Furthermore, in the same study, it was proven that patients with any of these comorbidities have a 5 to 6 times greater chance of flap necrosis.
In a systematic review that evaluated risk factors concerning pediculate flaps used in the lower limbs, it was observed that there was no significant increase in relative risk in smokers. However, smoking almost reached statistical significance as a risk factor3. In our study, smokers and diabetic patients were five times more likely to have partial necrosis of the flap.
Despite the occurrence of complications, most of the time, we conducted only with debridement, sterile dressing without adding medications and observation of the wound until its healing by second intention, which occurred within four weeks, and skin grafting is necessary in only one case. Similar conduct was also taken by other authors, who in all their cases treated partial necrosis conservatively3,5,10,15.
CONCLUSION
According to the results demonstrated here and in the literature, we can conclude that the sural flap is a good alternative for the coverage of lower limb lesions due to the good success rate with few complications. However, despite the advantages, this type of flap is not free of complications, but most of the times, they cause little morbidity and are easy to manage.
We should be aware of the risk factors of these complications such as diabetes, peripheral vascular disease, hypertension, and smoking; and, especially, when there is an association of factors in the same patient, such as diabetes and smoking, which increased the risk of partial necrosis of the flap by five times.
COLLABORATIONS
|
PSB |
Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Project Administration, Writing - Original Draft Preparation |
|
HA |
Final manuscript approval |
|
TSS |
Conception and design study, Final manuscript approval, Project Administration |
|
TSB |
Analysis and/or data interpretation, Conception and design study, Formal Analysis, Methodology |
REFERENCES
1. Singh KK, Rohilla R, Singh R, Singh S, Singh B, Tanwar M. Outcome of distally based sural artery flap for distal third of leg and foot defects. J Ayub Med Coll Abbottabad. 2017 Jul/Set;29(3):462-5.
2. Garcia AMC. Retalho sural reverso para reconstrução distal da perna, tornozelo, calcanhar e do pé. Rev Bras Cir Plást. 2009;24(1):96-103.
3. Dalponte M. Retalho sural para reconstrução do pé. Arq Catarin Med. 2007 Jun;36(Supl 1):1-4.
4. Masquelet AC, Romana MC, Wolf G. Skin island flaps supplied by the vascular axis of the sensitive superficial nerves: anatomic study and clinical experience in the leg. Plast Reconstr Surg. 1992 Jun;89(6):1115-21.
5. Severo AL, Coppi EFM, Cavalheiro HL, Dal Bosco AL, Barreto Filho D, Lemos MB. Reconstrução de membro inferior-retalho fasciocutâneo sural. Rev Bras Ortop. 2019 Mar/Abr;54(2):128-33.
6. Sauerbier M. Retalho sural. In: Moran LS, Cooney PW, eds. Cirurgia de tecidos moles: master techniques in orthopedic. São Paulo: Thieme Revinter; 2015.
7. Grandjean A, Romana C, Fitoussi F. Retalho sural de base distal para cobertura de tornozelo e pé em crianças. Ortop Traumatol Cirur Pesq. 2016;102(1):111-6.
8. D'Avila F, Franco D, D'Avila B, Arnaut Junior M. Use of local muscle flaps to cover leg bone exposures. Rev Col Bras Cir. 2014 Nov/Dez;41(6):434-9.
9. Dhamangaonkar AC, Patankar HS. Reverse sural fasciocutaneous flap with a cutaneous pedicle to cover distal lower limb soft tissue defects: experience of 109 clinical cases. J Orthop Traumatol. 2014 Set;15(3):225-9.
10. Vendramin FS. Retalho sural de fluxo reverso: 10 anos de experiência clínica e modificações. Rev Bras Cir Plást. 2012;27(2):309-15.
11. Quirino AC, Viegas KC. Retalhos fasciocutâneos para cobertura de lesões no pé e tornozelo. Rev Bras Ortop. 2014;49(2):183-8.
12. Parrett BM, Pribaz JJ, Matros E, Przylecki W, Sampson CE, Orgill DP. Risk analysis for the reverse sural fasciocutaneous flap in distal leg reconstruction. Plast Reconstr Surg. 2009 Mai;123(5):1499-504.
13. Ciofu RN, Zamfirescu DG, Popescu AS, Lascar I. Reverse sural flap for ankle and heel soft tissues reconstruction. J Med Life. 2017 Jna/Mar;10(1):94-8.
14. Baumeister SP, Spierer R, Erdmann D, Sweis R, Levin LS, Germann GK. A realistic complication analysis of 70 sural artery flaps in a multimorbid patient group. Plast Reconstr Surg. 2003 Jul;112(1):129-40;discussion:141-2.
15. Chang SM, Li XH, Gu YD. Distally based perforator sural flaps for foot and ankle reconstruction. World J Orthop. 2015 abr;6(3):322-30. Figura 2. A. Rotação e posicionamento do retalho sob a lesão; B. Pós-operatório imediato; C. Pós-operatório tardio.
1. Hospital Municipal São José, Joinville, SC, Brazil.
2. Joinville Institute of Orthopedics and Traumatology, Joinville, SC, Brazil.
3. Institute of Higher Education of Vale do Parnaíba, Parnaíba, PI, Brazil.
*Corresponding author: Pedro Simão Bosse, Rua Santo Antônio, nº 414, apto 601, Centro, Criciúma, SC, Brazil, Zip Code 88801-440. E-mail: pedrobosse@hotmail.com
Article received: December 14, 2020.
Article accepted: April 23, 2021.
Conflicts of interest: none.







 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter