

Original Article - Year 2021 - Volume 36 -
Brosco-Dutka classification system for palate fistulas
Sistema de classificação Brosco-Dutka para fístulas de palato
ABSTRACT
Introduction: The occurrence of post-palatoplasty oronasal fistula (ONF) is undesirable, challenging and difficult to classify complications. The objective is to present a classification protocol for palate fistula based on the fistula's morphological, embryological criteria and symptomatology.
Methods: The elaboration of the classification involved the following steps: definition of ONF; definition of anatomical references; establishment of embryological and morphological criteria; inclusion of symptomatology.
Discussion: The established protocol includes strategies for identifying anatomical references of complex visualization such as foramen (FI) and the transition area between the hard and soft palate. From the point of view of embryology, the fistula can be classified as PREFI (located in the region before the FI), POSFI (located in the region after the FI) and PREPO (which affects both the region before and after the FI). The morphological criterion establishes as areas: region-1: prealveolar and/or the alveolar arch; region-2: hard palate before FI; region-3: hard palate after FI; region-4: transition between hard and soft palate; and region-5: soft palate. Symptom identification includes hypernasality, ear infections and nasal reflux, in addition to asymptomatic fistulas. Obtaining adequate intraoral photographs facilitates the protocol's applicability, and the positioning for the photographic image requires the visualization of the palatal face of the upper incisor teeth.
Conclusion: The Brosco-Dutka protocol for the classification of palate fistula was developed for use by the craniofacial team during a face-to-face consultation or photographic image analysis. The proposal presents illustrations to guide the proper use of the criteria.
Keywords: Cleft palate; Oral fistula; Clinical protocols; Protocols; Plastic surgery.
RESUMO
Introdução: A ocorrência de fístula oronasal (FON) pós-palatoplastia é uma complicação indesejável, desafiadora e de difícil classificação. O objetivo é apresentar um protocolo de classificação de fístula de palato baseado em critérios morfológicos, embriológicos e sintomatologia da fístula.
Métodos: A elaboração da classificação envolveu as seguintes etapas: definição de FON; definição de referências anatômicas; estabelecimento de critérios embriológicos e morfológicos; inclusão da sintomatologia.
Discussão: O protocolo estabelecido inclui estratégias para a identificação de referências anatômicas de complexa visualização como forame incisivo (FI) e a área de transição entre o palato duro e mole. Do ponto de vista da embriologia, a fístula pode ser classificada como PREFI (localizada em região anterior ao FI), POSFI (localizada em região posterior ao FI) e PREPO (que acomete tanto a região anterior quanto posterior ao FI). O critério morfológico estabelece como áreas: região-1: pré-alveolar e/ou do arco alveolar; região-2: palato duro anterior ao FI; região-3: palato duro posterior ao FI; região-4: transição entre palato duro e mole; e região-5: palato mole. A identificação de sintomas inclui: hipernasalidade, otites e refluxo nasal, além das fístulas assintomáticas. A obtenção de fotografias intraorais adequadas facilita a aplicabilidade do protocolo, sendo que o posicionamento para imagem fotográfica requer a visualização da face palatina dos dentes incisivos superiores.
Conclusão: O protocolo Brosco-Dutka de classificação de fístula de palato, foi elaborado para uso pela equipe craniofacial em consulta presencial ou durante análise de imagens fotográficas. A proposta apresenta ilustrações para nortear o uso adequado dos critérios.
Palavras-chave: Fissura palatina; Fístula bucal; Protocolos clínicos; Protocolos; Cirurgia plástica
INTRODUCTION
In cases of cleft lip and palate (CLP), palatoplasty aims at the morphological and functional restoration of the palate, establishing a functional velopharyngeal mechanism for speech, swallowing and hearing, preserving the growth potential of the middle third of the face1,2,2. Post-palatoplasty complications may include hemorrhage, difficulty breathing, patch necrosis, repair dehiscence. The occurrence of oronasal fistula (ONF) and velopharyngeal dysfunction (VDP) is the most challenging interdisciplinary team complication. ONF may result from tension in the surgical repair of the palate, and its healing is accompanied by fibrosis compromising tissue vascularization. The healing process, therefore, produces retractions with morphological and functional consequences. The occurrence of ONF is associated with speech impairments, nasal reflux, halitosis and chronic infections, being an indicator of surgical success or failure3-8.
The magnitude of the occurrence of ONF is not fully known, and there are divergences regarding the indexes reported in the literature ranging from 0%9-11 to 78%12. The variation in ONF occurrence results from the lack of standardization of evaluation protocols and a definition of what should be considered fistula13,14. Papers on ONF of scientific relevance were published, including meta-analyses14, systematic reviews7,15 and systematic scope reviews16. However, most studies do not control variables that affect the occurrence of ONF, do not describe in detail the surgical techniques of primary palatoplasty, nor do they measure the amplitude of the cleft before surgery4,7,8,14,16,17. The lack of reports on criteria for the inclusion and exclusion of ONF in published studies is also an impact factor on the percentage of occurrence of these complications since some studies exclude fistulas before the incisor foramen or intentionally not repaired fistulas2,13,18-23.
Understanding the importance and complexity of the identification of ONF, some classification systems addressed both the standardization of the nomenclature13,18,24 and the surgical management of these occurrences24,25. These publications reflect the concern about the resolution of ONF seeking to favor the prevention and treatment of these complications. A study involving data analysis recorded in medical records of 466 patients with a unilateral transforaminal cleft, Jacob et al. (2020)26 reported both variabilities in terminology and a lack of consensus between the areas of plastic surgery and speech therapy regarding the occurrence of ONF. They were observed by Jacob et al. (2020)26: absent or incomplete records in medical records, inadequate photographic images for the identification of ONF and divergences regarding the inclusion of fistulas in the preforaminal incisive region as surgical complications. The authors indicated the need for a comprehensive fistula classification system, which can be used effectively by multidisciplinary teams, optimizing the systematic documentation of the results of the treatment of FLP26.
OBJECTIVE
The present study aimed to develop a protocol for the classification of palate fistula based on morphological, embryological and symptomatology criteria of the fistula.
METHODS
This study was conducted at the Craniofacial Anomalies Rehabilitation Hospital of the University of São Paulo (HRAC-USP), from 2013 to 2017, after approval by the Institution’s Ethics Committee (protocol no. 3.305124).
The development of the protocol occurred after the survey of 466 medical records of a randomized clinical trial involving patients with unilateral complete cleft lip and palate and 13,876 photographic images of patients of the institution, analyzing the presence and location of the ONF, reported by the areas of plastic surgery and speech therapy. The survey indicated the use of distinct terminology between the areas, failure to document the occurrences (incomplete or absent data), and inadequate photographic images for ONF classification. Considering the difficulties encountered, the planning for the construction of this protocol included the following steps: 1) to standardize the definition of ONF; 2) define the anatomical references; 3) establish embryological and morphological criteria; 4) include the symptomatology; and 5) establish criteria for photographic imaging.
Brosco-Dutka classification system
Definition of ONF
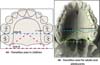
In this protocol, we defined a fistula as a failure to heal or rupture in the primary surgical repair of the palate, according to Cohen et al. (1991) 18 and Muzaffar et al. (2001) 27. Palate dehiscence was defined as the rupture of the surgical closure of an entire morphological region of the palate and classified using the same protocol (Figure 1). It is also postulated that all fistulas should be reported, including punctate fistulas (micro-fistulas), asymptomatic fistulas, and fistulas left intentionally.
Anatomical references
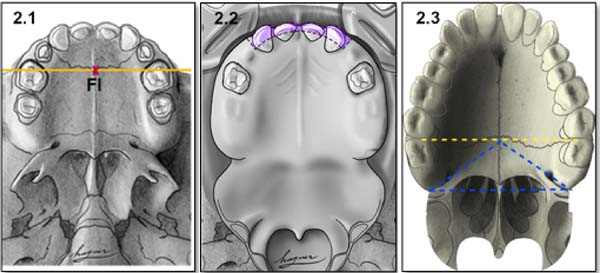
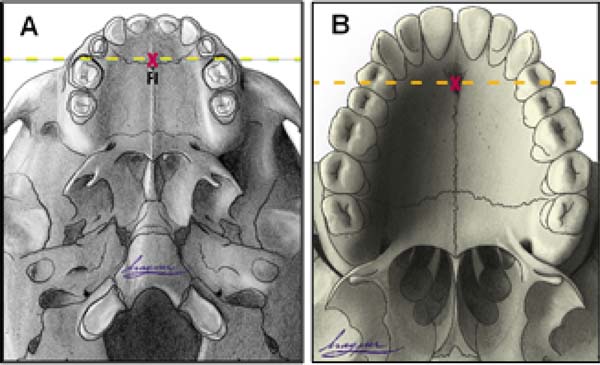
Anatomical references include incisive foramen, upper alveolar arch, and the transition area between the hard palate and soft palate (Figure 2).
Incisor foramen (FI)
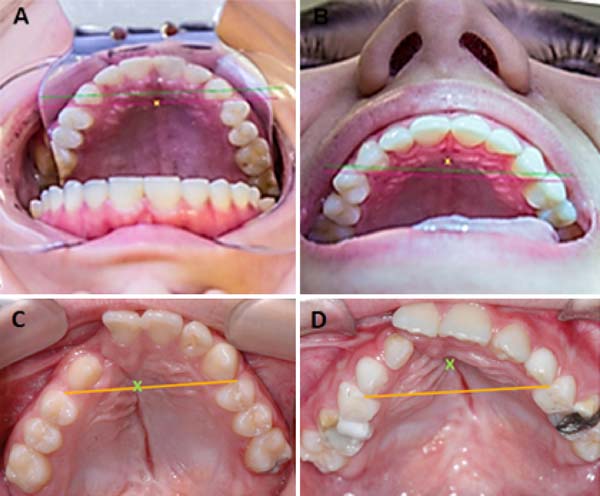
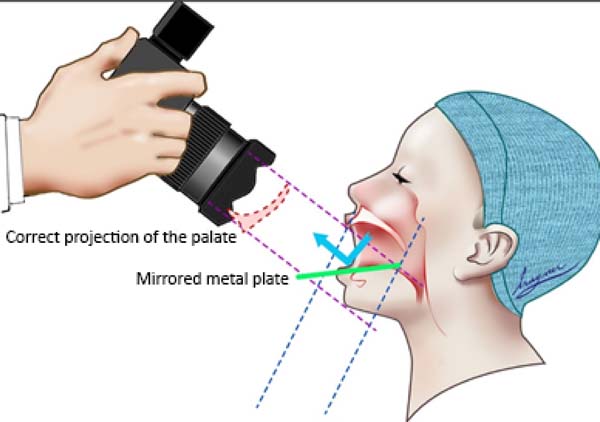
The FI is a demarcatory anatomical point that fragments the primary palate from the secondary palate; however, in patients with FLP, it is nonexistent due to the absence of bone at the cleft site24. Defining the transition point between the primary and secondary palate in patients with a history of FLP is a complex task, hampered by morphological changes inherent to the fissure and resulting from the fibrosis process associated with post-surgical healing. To favor this process, the Brosco-Dutka protocol establishes the incisive line as an imaginary line drawn between the points of contact between the canine teeth and the 1st deciduous molar in the child (Figure 3A). In adults, this line is drawn between the point of contact between the canines and the 1st premolar (Figure 3B). When considering the analysis of photographic images for the definition of FI, it is fundamental to standardize to obtain intraoral images. Photographic documentation must be obtained with the use of mirrors for photography by a trained professional.
Upper alveolar arch
The upper alveolar arch is the portion of the maxilla that lines the dental alveoli, being an important anatomical reference for identifying pre-alveolar fistulas and fistulas of the alveolar arch, which are those located in the region of the labial vestibule and the alveolar arch itself. The terminology “vestibular fistulas” or “oronasal fistulas” should not be used in this classification.
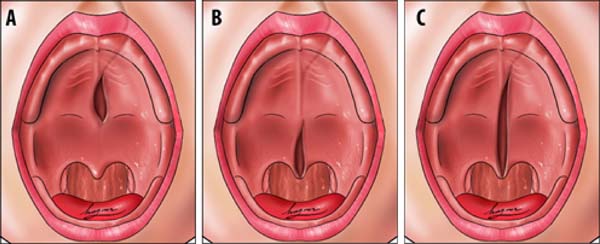
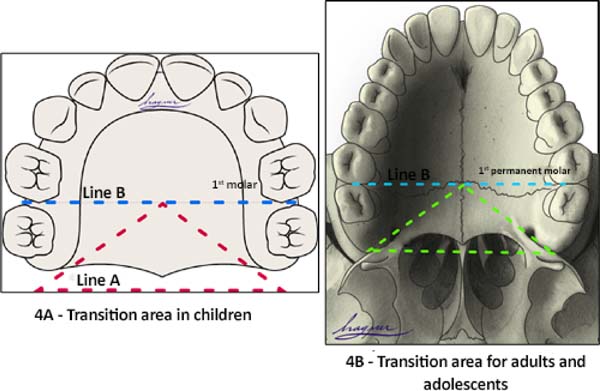
Transition area between the hard palate and soft palate
The transition area between the hard and soft palate is a region where many ONF occur because it is one of the areas of greatest tension during palatoplasty. The identification of ONF in this region is complex due to morphological changes resulting from the fistula healing process, resulting in retraction and anteriorization of the soft palate muscles, giving the impression that the transition fistula extends to the hard palate or soft palate. To favor the process of identification of the transition area between the hard palate and the soft, the Brosco-Dutka protocol establishes two imaginary lines as illustrated in Figure 4:
During the evaluation of the ONF, the transition area is that circumscribed to an imaginary triangle whose base is formed by line A and the apex is at the central point of line B. During the analysis of photographic images, if the photographic documentation is inadequate, the definition of the transition area becomes complex or even impossible.
Embryological and morphological criteria
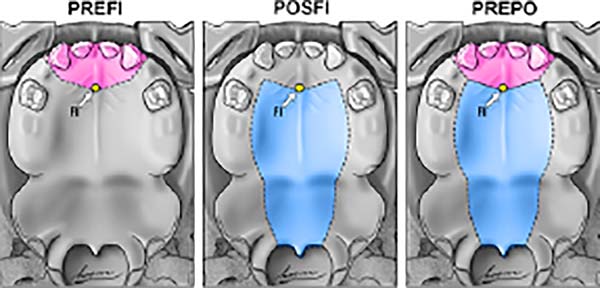
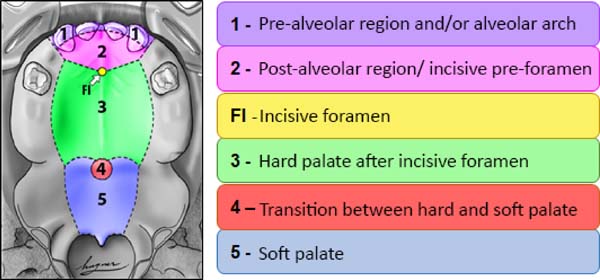
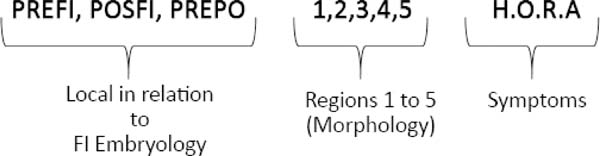
From the point of view of embryology, the fistula can be called fístula PREFI, POSFI or PREPO (Figure 5). Regarding morphology, the fistula may occur in Regions 1, 2, 3, 4 and 5 (Figure 6).
The embryological criterion analyzes the presence of the fistula in the primary and/or secondary palate, according to its location in relation to the FI (Figure 5). The initial stage of application of the protocol, therefore, requires the identification of the FI and the classification of the fistula in:
Then the morphological criterion is applied by verifying in which of the five regions the ONF occurred, being possible the involvement of more than one morphological region (Figure 6):
• Region 1: involves the pre-alveolar and/or alveolar arch;
• Region 2: involves the post-alveolar region (area of the hard palate anterior to FI)
• Region 3: involves the region of the hard palate after FI;
• Region 4: involves the transition region between the hard palate and the soft palate;
• Region 5: wraps the soft palate.
Symptomatology
The symptomatology can be observed by the professional during the clinical evaluation in person, reported by the patient or his/her guardian or obtained from records in the patient’s medical records. In the protocol, the word HORA was used to characterize the reported symptoms, as illustrated (Figure 7), where H refers to hypernasality and/or nasal air escape or other speech alteration; O refers to otitis and other otological symptoms; R refers to nasal reflux of food, and A refers to asymptomatic fistulas.
The coexistence of ONF and VFD makes the identification of speech-related symptoms and swallowing more complex. Whenever possible, face-to-face evaluation of symptoms should be performed under two conditions: with and without fistula filling. A fistula may be temporarily combed with a dental material, host, or adhesive to retain dental prosthesis. Even at the best of attempts, a fistula may not be fully sealed, which always leaves doubts about VFD’s coexistence. The elimination of symptoms with fistula sealing provides essential information for the definition of the conduct for fistula correction. In cases of VFD, fistula-only repair does not correct symptoms arising from velopharyngeal insufficiency. Instrumental evaluation of velopharyngeal functioning for the speech employing nasopharyngoscopy and/or videofluoroscopy is necessary to decide between operating only the fistula or associating this procedure with secondary intravelar veloplasty for muscle repositioning of the soft palate.
At the end of the fistula evaluation on face-to-face examination or when performing photographic image analysis in the Brosco-Dutka classification, the findings can be represented by a formula combining the embryological, morphological and symptomatology criteria (Figure 7).
Photographic documentation
To obtain good photographic images for applying the Brosco-Dutka protocol, it is important to use a mirror for the photograph of the intraoral region, positioned in adequate angulation in relation to the occlusal plane (between 45º and 60º). The photographic image should allow the visualization of the palatine face of the upper incisor teeth instead of the vision of the anterolabial face of the same. The image in Figure 8 illustrates the proper positioning of the mirror and camera during the photoshoot. The images of Figures 9A and 9B illustrate the appropriate photographic image (Figure 9A) and inadequate photographic taking (Figure 9B) in an individual without FLP. Figures 9C and 9D illustrate the appropriate (Figure 9C) and inadequate (Figure 9D) photo in an individual with FLP. For the complete photographic visualization of the palate, two photographic shots are required: using the mirror for the anterior region and direct take of the soft palate with or without the use of the mirror for the posterior region. With a single photograph, proper photographic viewing of the entire palate (hard and soft) is limited due to the configuration of the palate.
DISCUSSION
The incidence of ONF is considered one of the indicators of failure of primary surgical treatment of FLP6-8. Although the literature presents publications on the individual and institutional results of complications after palatoplasty, it is also observed the absence of a standardized and universally accepted definition of the concept of fistula13,14,19. Several definitions of fistulas have been used in the literature, including failure in healing or rupture of surgical repair of the palate18,27 or permeability between the oral and nasal cavity13,23,28,29. The absence of the standardized definition of fistula results in great variability of the occurrence rates of ONF 14, introducing biases and compromising the establishment of this index8.
The description of inclusion and exclusion criteria of ONF in publications is also limited, with great heterogeneity among researchers. Some exclude fistulas before FI and intentionally unrepaired, considering only fistulas on the secondary palate2,13,18-23. Documenting only fistulas on the secondary palate modifies the occurrence rate of ONF, and FI is the criterion applied; it should be clearly indicated in the publication.
A fistula classification system that can be applied with good reliability among team members is considered essential for adequate control of the variation of the ONF indices 8,13,18. The classification systems of Cohen18 and Pittsburgh13 were based on anatomical criteria and did not mention the symptoms. Sitzman et al. (2016) 8 evaluated the reliability of the Pittsburgh fistula classification system, involving eight surgeons as evaluators. The results indicated that the Pittsburgh classification showed good intra-rater reliability, but the inter-rater agreement rates were not as good as expected. Inadequate photographic documentation was one of the limiting factors cited by the authors.
The Richardson and Agni classification system (2014)25 proposes an algorithm for fistula management based on parameters that involve:
1)the size of the fistula (longitudinal or transverse); 2) the affected site (soft palate and uvula, posterior and middle hard palate, hard anterior palate); 3) classification of the cleft (unilateral, bilateral); and 4) number of previous surgical procedures on the palate. According to the authors, the proposed classification allows the surgeon to assess the degree of difficulty in correcting the fistula and predicting the prognosis of the procedure. More recently, a classification system for palate fistula was proposed by Fayyaz (2019)24. The authors published a very expressive series of 2,537 fistulas that were analyzed. The classification is based on four characteristics: 1) location of the fistula, 2) fistula size, 3) velopharyngeal competence, and 4) presence of dehiscence. When present, multiple fistulas were reported. The algorithm proposed by the authors makes it possible to establish guidelines for surgical management. Fayyaz (2019)24 mentions that the correction of velopharyngeal insufficiency is performed together with the correction of the fistula. Still, he does not report the use of nasoendoscopy and videofluoroscopy of velopharyngeal functioning during the process of defining the conduct for managing VFD.
The classification proposed in the present study, although similar to those proposed by Cohen et al. (1991) 18 and Smith et al. (2007) 13, includes five possible regions of occurrence instead of 7 and proposes criteria that favor the identification of anatomical references essential for the application of the new protocol. The introduction of the incisor line (to assist in locating the FI) and lines A and B (to assist in locating the transition area between hard and soft palate) aim to favor greater reliability among the evaluators during the application of the classification system. In cases with severe surgical sequelae involving bone deformities and significant loss of dental elements, this classification may not be completely applicable due to the absence of reference elements.
It is also proposed that this protocol be used to document fistulas intentionally left by the surgeon at the time of primary palatoplasty. In addition, asymptomatic fistulas and punctate fistulas (micro-fistulas) should be documented with a clear indication of their clinical condition of no relevance for speech. , food and hearing. Only with this care of registering all possible occurrences of fistula will future comparative studies be able to generate adequate scientific evidence of the actual occurrence of ONF, thus contributing to the prevention and treatment of these complications7.
The complete record of data on post-surgical complications should be inserted in the patient’s medical records in a protocol for evaluating these occurrences, being fundamental for adequate documentation of the institutional results of the management of the FLP. Obtaining quality photographic images that allow visualization of the complete palate is essential to verify reliability during protocol application. Different services may establish different strategies for intraoral photography, and it is possible to use the dental chair with a mirror or the surgical table with the patient anesthetized for the correction of the fistula. Image quality should be audited periodically, ensuring appropriate, photographic collections for future national and international intercenter studies. The team’s training (surgeon, dentist and speech therapist) for the protocol application is essential in the routine of specialized services, being recommended a periodic calibration and establishing strategies to improve or maintain intra and inter-rater reliability.
CONCLUSION
The Brosco-Dutka protocol of classification of the palate fistula has as its main purpose its use by a multidisciplinary team, aiming to enable both intra and craniofacial intercenters. Although it is possible to apply it in live outpatient consultations, it is recommended to obtain good photographic images of the palate so that the classification of post-surgical complications can be made by multiple evaluators internal and external to the craniofacial center evaluated. The classification of fistula by multiple evaluators, in turn, requires adequate photographic documentation, including documentation of fistula-related symptomatology.
The protocol defines what fistula is and standardizes the terminology to be used by the multidisciplinary team. The proposed classification is based on embryological and morphological criteria, including strategies for identifying anatomical references of complex visualization. Based on the care highlighted by the authors, the application of the protocol allows systematic and standardized monitoring of the occurrence of ONF, favoring the identification of the relevance of these complications for speech, hearing and feeding. The clinical validation of this protocol is necessary, and future studies involving its application should clarify the inclusion and exclusion criteria of ONF. It is essential to state in the methodology whether fistulas intentionally left by the surgeon (which are not considered surgical complications) and asymptomatic fistulas (which do not require surgical or prosthetic management), for example, will be computed in the general percentage of the occurrence.
REFERENCES
1. Silva Filho OG, Freitas JAS. Caracterização morfológica e origem embriológica. In: Trindade IEK, Silva Filho OG, coordenadores. Fissuras labiopalatinas: uma abordagem interdisciplinar. São Paulo: Santos; 2007. p. 17-49.
2. Williams WN, Seagle MB, Pegoraro-Krook MI, Souza TV, Garla L, Silva ML, et al. Prospective clinical trial comparing outcome measures between Furlow and von Langenbeck palatoplasties for UCLP. Ann Plast Surg. 2011 Fev;66(2):154-63.
3. Deshpande GS, Campbell A, Jagtap R, Restrepo C, Dobie H, Keen HT, et al. Early complications after cleft palate repair: a multivariate statistical analysis 709 of patients. J Craniofac Surg. 2014;25(5):1614-8.
4. Passos VAB, Carrara CFC, Dalben GS, Costa B, Gomide MR. Prevalence, cause and location of palatal fistula in operated complete unilateral cleft lip and palate: retrospective study. Cleft Palate Craniofac J. 2014 Mar;51(2):158-64.
5. Aslam M, Ishaq I, Malik S, Fayyaz GQ. Frequency of oronasal fistulae in complete cleft palate repair. J Coll Physicians Surg Pak. 2015 Jan;25(1):46-9.
6. Eberlinc A, Koželj V. Incidence of residual oronasal fistulas: a 20-year experience. Cleft Palate Craniofac J. 2012 Nov;49(6):643-8.
7. Hardwicke JT, Landini G, Richard BM. Fistula incidence after primary cleft palate repair: a systematic review of the literature. Plast Reconstr Surg. 2014 Out;134(4):618e-27e.
8. Sitzman TJ, Allori AC, Matic DB, Beals SP, Fisher DM, Samson TD, et al. Reliability of oronasal fistula classification. Cleft Palate Craniofac J. 2018 Jul;55(6):871-5.
9. Xu JH, Chen H, Tan WQ, Lin J, Wu WH. The square flap method for cleft palate repair. Cleft Palate Craniofac J. 2007 Nov;44(6):579-84.
10. Stewart TL, Fisher DM, Olson JL. Modified Von Langenbeck cleft palate repair using an anterior triangular flap: decreased incidence of anterior oronasal fistulas. Cleft Palate Craniofac J. 2009 Mai;46(3):299-304.
11. Dong Y, Dong F, Zhang X, Hao F, Shi P, Ren G, et al. An effect comparison between Furlow double opposing Z-plasty and two-flap palatoplasty on velopharyngeal closure. Int J Oral Maxillofac Surg. 2012 Mai;41(5):604-11.
12. Mak SY, Wong WH, Or CK, Poon AMS. Incidence and cluster occurrence of palatal fistula after furlow palatoplasty by a single surgeon. Ann Plast Surg. 2006 Jul;57(1):55-9.
13. Smith DM, Vecchione L, Jiang S, Ford M, Deleyiannis FWB, Haralam MA, et al. The Pittsburgh fistula classification system: a standardized scheme for the description of palatal fistulas. Cleft Palate Craniofac J. 2007 Nov;44(6):590-4.
14. Bykowski MR, Naran S, Winger DG, Losee JE. The rate of oronasal fistula following primary cleft palate surgery: a meta-analysis. Cleft Palate Craniofac J. 2015 Jul;52(4):e81-7.
15. Timbang MR, Gharb BB, Rampazzo A, Papay F, Zins J, Doumit G. A systematic review comparing Furlow opposing Z-plasty and straight-line intravelar veloplasty methods of cleft palate repair. Plast Reconstr Surg. 2014 Nov;134(5):1014-22.
16. Salimi N, Aleksejuniene J, Yen EHK, Loo AY. Fistula in cleft lip and palate-a systematic scoping review. Ann Plast Surg. 2017 Jan;78(1):92-102.
17. Hardwicke J, Nassimizadeh M, Richard B. Reporting of randomized controlled trials in cleft lip and palate: a 10-year review. Cleft Palate Craniofac J. 2017 Mar;54(2):142-52.
18. Cohen SR, Kalinowski J, LaRossa D, Randall P. Cleft palate fistulas: a multivariate statistical analysis of prevalence, a etiology and surgical management. Plast Reconstr Surg. 1991 Jun;87(6):1041-7.
19. Emory RE, Clay RP, Bite U, Jackson IT. Fistula formation after palatal closure: an institutional perspective. Plast Reconstr Surg. 1997 Mai;99(6):1535-8.
20. Diah E, Lo LJ, Yun C, Wang R, Wahyuni LK, Chen YR. Cleft oronasal fistula: a review of treatment results and a surgical management algorithm proposal. Chang Gung Med J. 2007 Nov/Dez;30(6):529-37.
21. Phua YS, Chalain T. Incidence of oronasal fistulae and velopharyngeal insufficiency after cleft palate repair: an audit of 211 children born between 1990 and 2004. Cleft Palate Craniofac J. 2008 Mar;45(2):172-8.
22. Losken HW, Van Aalst JA, Teotia SS, Dean SB, Hultman S, Uhrich KS. Achieving low cleft palate fistula rates: surgical results and techniques. Cleft Palate Craniofac J. 2011;48(3):312-20.
23. Rossell-Perry P, Segura E, Salas-Bustinza L, Cotrina-Rabanal O. Comparison of two models of surgical care for patients with cleft lip and palate in resource-challenged settings. World J Surg. 2015 Jan;39(1):47-53.
24. Fayyaz GQ, Gill NA, Ishaq I, Aslam M, Chaudry A, Ganatra MA, et al. Pakistan comprehensive fistula classification: a novel scheme and algorithm for management of palatal fistula/dehiscence. Plast Reconstr Surg. 2019 Jan;143(1):140e-51e.
25. Richardson S, Agni NA. Palatal fistulae: a comprehensive classification and difficulty index. J Maxillofac Oral Surg. 2014 Set;13(3):305-9.
26. Jacob MF, Prearo GA, Brosco, TVS, Silva, HLA, Dutka, JCR. Fístula após palatoplastia primária: Consenso entre profissionais da cirurgia plástica e da fonoaudiologia. Rev Bras Cir Plást. 2020;35(2):142-8.
27. Muzaffar AR, Byrd HS, Rohrich RJ, Johns DF, LeBlanc D, Beran SJ, et al. Incidence of cleft palate fistula: an institutional experience with two-stage palatal repair. Plast Reconstr Surg. 2001 Nov;108(6):1515-8.
28. Rennie A, Treharne LJ, Richard B. Throat swabs taken on the operating table prior to cleft palate repair and their relevance to outcome: a prospective study. Cleft Palate Craniofac J. 2009 May;46(3):275-9.
29. Lu Y, Shi B, Zheng Q, Hu Q, Wang Z. Incidence of palatal fistula after palatoplasty with levator veli palatini retropositioning according to Sommerlad. Br J Oral Maxillofac Surg. 2010 Dez;48(8):637-40.
30. Brosco TVS. Fístula de palato após reparo da fissura labiopalatina em um estudo clínico randomizado [tese]. Bauru (SP): Universidade de São Paulo - Hospital de Reabilitação de Anomalias Craniofaciais; 2017.
31. Silva HLA. Atlas de cirurgia plástica na fenda labiopalatal [dissertação]. São Paulo (SP): Universidade de São Paulo - Hospital de Reabilitação de Anomalias Craniofaciais; 2019.
1. Craniofacial Anomalies Rehabilitation Hospital of the University of São Paulo -
HRAC-USP, Bauru, SP, Brazil.
2. University of São Paulo, Bauru Dental School, Bauru, SP, Brazil.
Corresponding author: Telma Vidotto de Sousa Brosco, Rua Sílvio Marchione, 3-20, Vila Nova Cidade Universitária, Bauru, SP, Brazil. Zip Code: 17012-900 E-mail: telmabrosco@gmail.com
Article received: July 21, 2020.
Article accepted: April 23, 2021.
Conflicts of interest: none
COLLABORATIONS
TVSB Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Formal Analysis, Methodology, Project Administration, Writing - Original Draft Preparation
GAP Analysis and/or data interpretation, Data Curation, Final manuscript approval, Methodology, Writing - Original Draft Preparation
HLAS Data Curation, Final manuscript approval, Methodology, Writing - Review & Editing
JCRD Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Formal Analysis, Methodology, Project Administration, Supervision, Writing - Original Draft Preparation






 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter