

Original Article - Year 2021 - Volume 36 -
Prospective study of inflammatory response in patients submitted to abdominoplasty after bariatric surgery
Estudo prospectivo da reposta inflamatória em pacientes submetidas à abdominoplastia pós-cirurgia bariátrica
ABSTRACT
Introduction: Obesity is a chronic inflammatory disease associated with changes in inflammatory markers such as interleukins and CRP. This study evaluates the inflammatory response, through variations in interleukins and CRP, in patients undergoing abdominoplasty.
Methods: Fourteen patients underwent abdominoplasty after weight loss achieved through bariatric surgery to maintain weight loss for at least18 months. Il4, IL6, IL10 and PCR levels were verified at times: preoperative, during surgery, 24 hours after surgery, 7th postoperative day and 14th postoperative day.
Results: IL4 increased in the 24 hours postoperatively and continued on the rise until the 14th day. IL10 went up during surgery and began to fall in the 24 hours postoperatively to levels lower than the initial ones. IL6 began to rise during surgery, being more expressive in the 24 hours postoperatively, followed by a fall until the 14th day. CRP increased 24 hours postoperatively and remained discharged until the 14th day.
Conclusion: Abdominoplasty reduced the chronic inflammatory systemic condition.
Keywords: Obesity; Plastic surgery; Abdominoplasty; Inflammation; Inflammation Mediators; Bariatric surgery.
RESUMO
Introdução: A obesidade é uma doença inflamatória crônica associada a alterações de marcadores inflamatórios como as interleucinas e PCR. O objetivo deste estudo é avaliar a resposta inflamatória, através das variações das interleucinas e PCR, em pacientes submetidas à abdominoplastia.
Métodos: Quatorze pacientes foram submetidas à abdominoplastia após perda ponderal alcançada por meio da cirurgia bariátrica com manutenção da perda ponderal por, ao menos, 18 meses. Os níveis de IL4, IL6, IL10 e PCR foram verificados nos tempos: pré-operatório, durante a cirurgia, 24 horas após a cirurgia, 7o dia pós-operatório e 14o dia pós-operatório.
Resultados: IL4 aumentou nas 24 horas de pós-operatório e seguiu em ascensão até o 14 o dia. IL10 subiu durante a cirurgia e começou a cair nas 24 horas de pós-operatório a níveis inferiores aos iniciais. IL6 começou a subir durante a cirurgia, mais expressivamente nas 24 horas de pós-operatório, seguida de queda até o 14o dia. A PCR aumentou nas 24 horas de pós-operatório e continuou alta até o 14o dia.
Conclusão: A abdominoplastia promoveu uma amenização do quadro inflamatório sistêmico crônico.
Palavras-chave: Obesidade; Cirurgia plástica; Abdominoplastia; Inflamação; Mediadores da Inflamação; Cirurgia bariátrica
INTRODUCTION
Obesity is an inflammatory disease1, characterized by the production of cytokines, concerning inflammatory responses2-4. These cytokines are interleukins (IL) produced in fatty tissue thanks to their endocrine properties. Thus, the inflammatory response of obesity causes increased production of inflammatory interleukins and reduces interleukins with anti-inflammatory properties5. The variation of these interleukins promotes a change in glycemic and insulinemic profile, characterizing a concomitant metabolic syndrome6,7.
OBJECTIVE
This study analyzes the variations in the inflammatory response through changes in interleukin levels and consequent modification of C-reactive protein (CRP) rates of post-bariatric patients submitted to anchor abdominoplasty.
METHODS
The present prospective study was carried out with fourteen female patients who underwent anchor abdominoplasty8 after weight loss obtained through bariatric surgery. The inclusion criteria were: presenting weight loss at the expense of bariatric surgery and maintaining the new weight for at least 18 months; being followed up in the body contouring surgery group at the Hospital de Clínicas of FMUSP during the period of the work, the first semester of 2018; in multidisciplinary follow-up, composed of an endocrinologist, digestive tract surgeon, psychologist and plastic surgeon. Exclusion criteria were: patients who were smokers, used contraceptive or replacement hormones, recreational drugs, or that could affect behavior.
The average age at the time of plastic surgery was 45.92 years, with extremes ranging from 29 to 60. The average interval between bariatric surgery and abdominoplasty was 5.8 years, ranging from 2 to 12 years. The mean BMI (body mass index) before bariatric surgery was 45.63 kg/m2, and before abdominoplasty, it was 29.62 kg/m2, with extremes of 21.3 to 36.7 kg /m2. The average weight of the resected surgical pieces was 2,068kg, varying between 1,000 and 3,600kg (Tables 1 and 2).
| Patients' age (years): | |
|---|---|
| Before bariatric surgery | Before abdominoplasty |
| Average 42 - 35 | Average 45-95 |
| Minimum 21 | Minimum 29 |
| Maximum 58 | Maximum 60 |
Blood samples were collected five times: preoperative, intraoperative, 24 hours after surgery,7 hours postoperatively and 14 th postoperative day.
Quantitative analyses of interleukins IL4, IL6 and IL10 were performed using the immunoassay method with magnetic beads Milliplex and MagPix System (Merck Millipore, USA). The results were measured in pg/dL (picograms per deciliter).
The C-reactive protein (CRP) was analyzed by C- Reactive Protein Gen.3. Immunoturbidimetric test for quantitative in vitro determination of PCR in human serum and plasma, using Roche/Hitachi cobas c systems and measured in mg/dL.
The patients signed a free and informed consent form authorizing the study before the procedure.
The project was approved by the hospital’s research ethics committee(CAPPesq)under number 48112015.8.0000.0068. It has no support from research funding agencies or private entities, so there is no conflict of interest.
RESULTS
The results obtained were grouped into absolute values and are presented in Table3.
| pre | trans | 12:00 p.m. | 7PO | 14PO | |
|---|---|---|---|---|---|
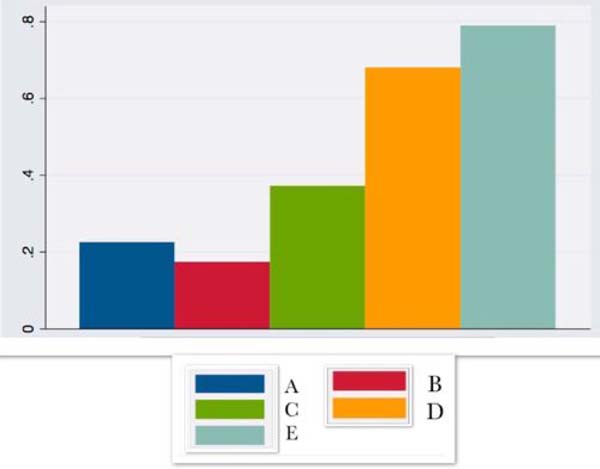
| IL4 | 0.22 ± 0.4 | 0.17 ± 0.2 | 0.37 ± 1.0 | 0.68 ± 1.2 | 0.79 ±2.2 |
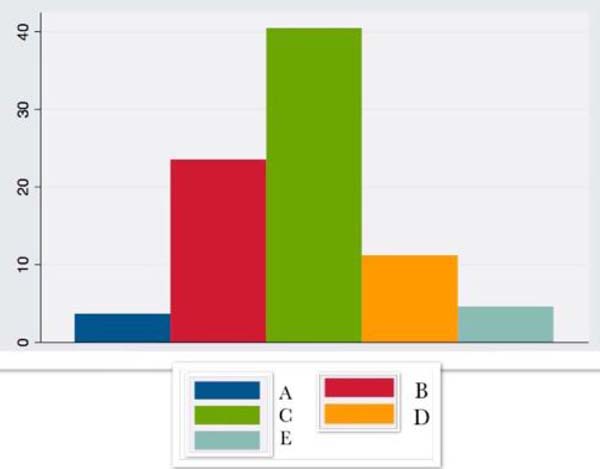
| IL6 | 3.66 ± 13.0 | 23.5 ± 23.9 | 40.43 ±15.1 | 11.2 ± 8.4 | 4.53 ± 8.5 |
| IL10 | 6.02 ± 6.2 | 47.4 ± 50.6 | 11.48 ± 6.9 | 6.18 ± 6.3 | 4.59 ± 4.2 |
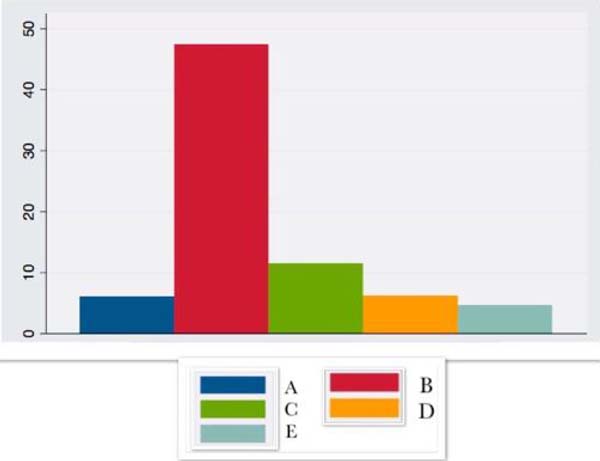
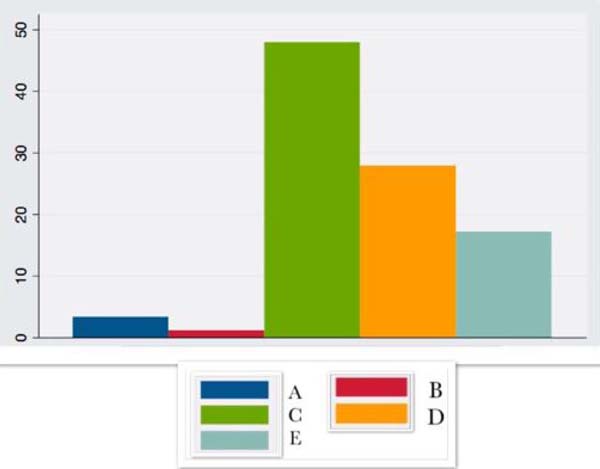
| CRP | 3.3 ± 7.7 | 1.15 ± + - 1.3 | 47.9 ±24.1 | 27.85 ± 19.8 | 17.2 ± 38.6 |
These results have graphic representation in Figures 1, 2, 3 and 4, where the preoperative (A), transoperative (B), 24-hour after surgery (C), 7 postoperative (D) and 14 postoperative (E) periods are presented.
IL4 began to rise in the first hours after surgery and remained on the rise until the 14th postoperative day.
IL6 increased intraoperatively, being more expressive in the 24 hours after surgery, with a progressive decrease until the 14thpostoperative day.
IL10 increased intraoperatively, followed by a fall lower than the initial levels.
CRP showed a large increase in the first hours after surgery, remaining elevated until the 14th postoperative day.
DISCUSSION
Fatty tissue is responsible for the production of interleukin IL6, considered an inflammatory marker, and IL4 and IL10, anti-inflammatory2-4. IL6, in addition to other properties, stimulates the production of inflammatory proteins in the liver, with special emphasis, the object of this study, of C-reactive protein (CRP). The patients in the study, being patients with severe obesity - mean BMI of 45.63 kg/m2 (35.6 to 56.7 kg/m2) - before bariatric surgery, despite having presented large weight losses, still maintained BMI elevated at the time of abdominoplasty, corresponding to 29.62 kg/m2 (21.3 to 36.7 kg/m2); therefore, with a basal pattern of inflammatory markers still high, as they are moderately obese. However, it is notorious that the weight loss induced by bariatric surgery, in addition to reducing diseases related to excess weight, minimizes the inflammatory state in morbidly obese patients7-10.
According to the literature 2,11-13, IL4, with anti-inflammatory activity, is produced by interacting the monocyte/macrophage complex with the adipose tissue. In response to the trauma, it begins an upward curve in the first hours of the postoperative period and remains on the rise until the 14th postoperative day.
Patients with compulsions can be considered to have a low degree of chronic inflammation, associated with increased inflammatory markers such as IL6 and CRP14,15, which have a considerable increase in the immediate intra and postoperative period, corresponding to the inflammatory response to surgical trauma16. CRP, in turn, presents an already high baseline value related to obesity itself, even with surgically achieved weight loss10, remaining elevated until the 14th postoperative day in the present study or for up to six months, according to other authors10,17.
IL10, an anti-inflammatory cytokine produced by adipocytes, increased intraoperatively, followed by a fall to levels lower than the initial ones. This fact agrees with the bibliography of 12,18 and reveals an anti-inflammatory reaction that accompanies the manifestations of IL6, considered antagonistic and whose inflammatory activity changes in the estimated period.
A abdominoplastia pós-cirurgia bariátrica não se limita a um melhor resultado estético e qualidade de vida, como já avaliado por outros autores19,20; sobretudo promove modificação dos marcadores inflamatórios e anti-inflamatórios, que, dada a ressecção do tecido gorduroso, acarretam uma amenização do quadro inflamatório crônico.
CONCLUSION
Patients with severe obesity, despite large weight losses at the expense of bariatric surgery, tend to persist with excess dermal fat tissue and, therefore, elevated baseline inflammatory and anti-inflammatory markers that, in response to surgical trauma, change for more time.
AGRADECIMENTOS
To Prof. Lineu Tadeu Velasco - Full Professor of the Discipline of Clinical Emergencies of the Department of Clinical Clinic of the Faculty of Medicine of the University of São Paulo - in whose laboratory - LIM-51, allowed the performance of the dosages of the interleukins present in work.
REFERENCES
1. Gallo JRB, Maschi-Signorini LB, Cabral CRB, Zuccari DAPC, Nogueira ML, Bozzola AR, et al. Skin protein profile after major weight loss and its role in body contouring surgery. Plast Reconstr Surg Glob Open. 2019 Ago;7(8):e2339. DOI: https://doi.org/10.1097/GOX.0000000000002339
2. Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013 Jun;17(6):851-9. DOI: https://doi.org/10.1016/j.cmet.2013.05.008
3. Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini- review. Gerontology. 2009;55(4):379-86.
4. Hotamisligil GS. Inflammation and metabolic desorders. Nature. 2006;444(7121):860-7.
5. Bulló M, Casas-Agustench P, Amigó-Correig P, Aranceta J, Salas-Salvadó J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. 2007 Out;10(10A):1164-72.
6. Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J Parenter Enteral Nutr. 2008 Nov/Dez;32(6):638-44.
7. Modolin MLA, Wilson Junior C, Rocha RI, Camargo CP, Giuliani NR, Gemperli R. Analysis of inflammatory and metabolic bomarkers in pacientes submetted to abdominoplasty after bariatric surgery. Acta Cir Bras. 2019;34(5):e201900506. DOI: http://dx.doi.org/10.1590/s0102-86502019005000000
8. Correa-Iturraspe M. Tratamiento quirurgico de la obesidad. Rev Assoc Med Argent. 1952,66:340-5.
9. Freitas Junior WR, Oliveira LVF, Perez EA, Ilias EJ, Lottenberg CP, Silva AS, et al. Systemic inflammation in severe obese patients undergoing surgery for obesity and weight-related diseases. Obes Surg. 2018;28:1931-42. DOI: https://doi.org/10.1007/s11695-017-3104-9
10. Chiappetta S, Schaack HM, Wölnerhannsen B, Stier C, Squillante S, Weiner RA. The impact of obesity and metabolic surgery on chronic inflammation. Obes Surg. 2018;28:3028-40. DOI: https://doi.org/10.1007/s11695-018-3320-y
11. Sippel C, Bastian RMA, Giovanella J, Faccin C, Contini V, Dal Bosco SM. Processos inflamatórios da obesidade. RAS. 2014 Out/Dez;12(42):48-56.
12. Gómez FI, Ortega MG, Alonso AA, Soler IO, Tafalla SA, Paredes MP, et al. Obesity, endothelial function and inflammation: the effects of weight loss after bariatric surgery. Nutr Hosp. 2016 Nov;33(6):1340-6.
13. Kohen VL, Candela CG, Fernández CF, Rosa LZ, Milla SP, Urbieta M, et al. Parámetros hormonales e inflamatorios en un grupo de mujeres com sobrepeso/obesidad. Nutr Hosp. 2011;26(4):884-9.
14. Arismendi E, Rivas E, Agustí A, Rios J, Barreiro J, Vidal J, et al. The systemic inflammome of severe obesity before and after bariatric surgery. Obes Sist Inflamm. 2014;9(9):e107859.
15. Lasselin J, Magne E, Beau C, Ledaguenel P, Dexpert S, Aubert A, et al. Adipose inflammation in obesity: relationship with circulating levels of inflammatory markers and association with surgery-induced weight loss. J Clin Endocrinol Metab. 2014 Jan;99(1):E53-61. DOI: https://doi.org/10.1210/jc.2013-2673
16. Barnes MA, Carson MJ, Nair MG. Non-traditional cytokines: how catecolamines and adipokines influence macrofages in immunity, metabolismo and central nervous system. Cytokine. 2015;72(2):210-9.
17. Hanusch-Enserer U, Cauza E, Spak M, Dunky A, Rosen HR, Wolf H, et al. Acute-phase response and immunological markers in morbid obese patients and patients following adjustable gastric banding. Int J Obes. 2003 Mar;27(3):355-61. DOI: https://doi:10.1038/sj.ijo.802240
18. Pamanicolau DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998 Jan;128(2):127-37.
19. Wilson Junior C, Modolin MLA, Gemperli R, Gobbi CIC, Faintuch J, Ferreira MA. Quality of life after abdominoplasty in women after bariatric surgery. Obes Surg. 2008 Jun;18(6):728-32.
20. Modolin MLA, Wilson Junior C, Rocha RI, Faintuch J, Camargo CP, Gemperli R. Quality of life after postbariatric abdominoplasty in females: interest of age, current weight and weight loss. J Obes Weight Loss Ther. 2015;5:272.
1. Hospital das Clínicas de São Paulo, Body Contour Group of the Plastic Surgery Discipline
of HC-USP, São Paulo, SP, Brazil.
2. Hospital das Clínicas of USP, Full Professor of the Plastic Surgery Service of
HC-USP, São Paulo, SP, Brazil.
Corresponding author: Nádia de Rosso Giuliani, Rua Capitão Rosendo, 100, Apt. 23b, Vila Mariana, São Paulo, SP, Brazil. Zip Code: 04120-060 E-mail: nagiuliani@yahoo.com.br
Article received: August 19, 2020.
Article accepted: April 23, 2021.
Conflicts of interest: none
SUPERVISION
NRG Analysis and/or data interpretation, Conception and design study, Formal Analysis, Methodology, Realization of operations and/or trials, Writing - Review & Editing
MM Final manuscript approval, Supervision
WCJ Supervision, Writing - Review & Editing
RIR Supervision, Writing - Review & Editing
RG Supervision









 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter