

Articles - Year 1998 - Volume 13 -
Autologous Fat Graft in Rabbits
Enxerto de Gordura em Coelhos
ABSTRACT
The aim of this study was to assess the fate of autologous fat tissue grafts harvested and processed by five different methods: blunt suction, sharp suction, dissection, treatment, and lavage. Fat was harvested from the subcutaneous tissue of the interscapular region in 25 rabits and injected into the subcutaneous area of the convex surface of the ear. Harvesting was by either open surgical excision. Where open surgical excision was used, the frgmants were cut into smaller pieces with scissors. Suction in the blunt and sharp suction groups was performed using cannulas with blunt or sharp edged suction holes, respectively. In both suction groups, the harvested tissue was injected without further processing. In the treatment group, 2 ml of cell 199 culture medium and Earle's basic salt solution were added, and in the lavage group the tissue was washed with Ringer's lactate. Animals were killed at 7 (n=9), 180 (n=8), and 360 (n=8) days. Serial cross sections were taken From each recipient area and the specimens processed for histology. The images from each section were digitalized in a computer and, with the assistence of image analysis softwere, the volume of remaining fat cells was calculated for each recipient area. The percentage volumes of fat cells found in each group at 360 days were: blunt suction 14,0%, sharp suction 35%, dissection 45%, treatment 27%, and lavage 16% (p=0.003).
Keywords: transplantation; graft; adipose tissue; graft survival; tissue survival.
RESUMO
A pesquisa consistiu em analisar o transplante livre de tecido adiposo autóloqo, em coelhos, obtido por 5 difirentes modalidades de colheita e preparo, a saber: aspirado, corte, dissecado, tratado e lavado. Nas duas primeiras modalidades, o tecido adiposo autólogo foi colhido por sucção. O aspirado (A) foi colhido com cânula de orifícios de bordas rombas e o corte (C) de bordas afiadas. As três demais modalidades de tecido adiposo foram obtidas por dissecção a céu aberto e fragmentação com tesoura delicada. O tecido assim colhido, fragmentado e injetado chamou-se dissecado (D). O tratado (T) recebeu adição de 2ml de meio de cultura de células 199 com sais de Earle. O lavado (L) foi submetido a lavagem com Ringer-lactato. As áreas doadoras do tecido adiposo foram as bolsas subcutâneas interescapulares. As orelhas dos coelhos foram as receptoras. Os animais foram sacrificados com 7 dias (9 animais) 180 (8 animais) e 360 dias (8 animais). Todas as áreas de transplante foram seccionadas transversalmente a cada 1 cm. A superfície de secção foi medida em sistema integrado para análise de imagem e cálculo do volume. Os cortes histopatolágicos foram quantificadas em: adipócitos, estroma, regiões císticas e inflamação. O volume percentual de adipócitos remanescentes nas 5 modalidades revelou aos 360 dias: Aspirado - 13,9%; Corte - 34,6%; Dissecado - 45,2%; Tratado - 27,0%; Lavado - 16,4%, com p = 0,003.
Palavras-chave: tecido adiposo; transplante autólogo; cirurgia plástica
Suction lipectomy has caused renewed interest in autologous fat grafts(12). Although it is an apparently simple method in which fat can be retricved and used for grafting, wide variations exist in harvesting and processing techniques, form of injection, rate of fat resorption, and other factors. There is therefore controversy about its value.
Clinical studies are of limited value for the assessment of the efficacy of fat grafting, as it is difticult to analyze preoperative and postoperative photographs objectively(7). Experimental studies, on the other hand, either try to evaluate too small volume of graft (around 1 ml)(5, 6, 10, 16, 20) or they score their findings subjectively(4) - which precludes quantitative analysis of gafrat, survival. The result is heterogeneous findings, so we were stimulated to pursue a more objective quantitative analysis, combining different methods of harvesting and processing.
MATERIAL & METHODS
Fat was harvested from the subcutaneous tissue of the inter scapular region of 25 male, New Zealand white rabbits, 6 months old with a average weight of 4,6 kg. The method used for harvesting was suction on one side and open surgical excision on the other, and assignment was random. This fat was then processed and injected as an autograft. Based on the method of harvesting and processing tive different types of grafting, were detined: blunt suction, sharp suction, dissection, treatment, and lavage.
Harvesting was by suction or open surgical excision. When open surgical excision was used, the fragments were cut into smaller pieces (about 4 mm) with sharp scissors.
Suction in the blunt and sharp suction groups was through cannulas with blum or sharp edged suction holes, respectively. In both instances, the internal diameter of the cannulas for suction as well as for injection was 5 mm, In both suction and dissection groups, the tissue harvested was placed into a syringe and immediately injected without further processing. In the treatment group, 2 ml of cell 199 culture medium and Earle's basic salt solution were added, and in the lavage group the tissue was washed over a screen with Ringer's lactate before injection.
The recipient site was the subcutaneous area of the convex surface of the rabbit's ear. A total of 5 ml of fat tissue was injected into each site, forming a cylindrical deposit along the route of injection. Each rabbit was grafted with all five of different methods, randomly distributed on both ears. The injection, which was done by manual compression of the syringe containing the fat, offered no resistance. Antibiotics were not used.
Animals were killed at 7 (n=9), 180 (n=8), and 360 (n=8) days. All five specimens were harvested from each animal. Each specimen was cut transversally by every 1cm (Fig. 1), resulting a mean of six fragments from each specimen. These fragments were fixed in 10% buffered formalin and embedded in paraffin for histological sections, which were stained with haematoxylin and eosin.
Fig. 1 Drawing of an car showing standard transverse cuts made in each specimen at a distance of 1 cm.
Morphometric Analysis
Four animals in each of the three time periods were randomly chosen for analysis. The presence of fat cells, stroma, cystic formation, and inflammatory reaction was scored in all histological sections. A reticle in a light microscope at magnification of 100 was used to quantify the amount in each process.
The areas that corresponded to a certain process were counted as points and the sum of those points was the score for that process. The average number of points counted for a whole cylindrical specimen was 3,503. The counts in each specimen were then transformed into percentages of each proeess.
Four specimens of fat tissue were harvested and immediately processed for histological examination to assess the amount of far cells and stroma present in the tissue before injection. Scoring was done in the same way.
Volumetric Calculation
The images from the histological sections of every specimen from all the animals were digiralized in a computer. With the help of image analysis software, the area occupied by fat cells and the total area of the graft were calculated. The volume of these elements was then calculated using the following formula:
where V = volume (mm3); d = distance between sections (mm); Σ(a) = sum of the areas (mm2); and k = constant of tissue retraction.
Determination of the Constant (k)
On 16 randomly chosen specimens, the areas of the cross sections were demarcated on a filter paper, before processing for histological examination.
These images on the paper filter were also digitalized in the computer and the areas obtained from fresilly sectioned tissue divided by the sum of the areas obtained after processing determined the constant of retraction (k).
To calculate the percentage volume of surviving fat cells the fallowing formula was used:

The data obtained from the calculations of the volume of graft and fat cells and absolute amounts of fat cells, stroma, cystic formation, and inflammatory reaction were compared by the Friedman two way analysis of variance by ranks. A probability (a) of less than 0.1 was accepted as signiticant.
RESULTS
Hyperaemia and edema were noticed in the ear of every rabbit on tht third day after grafting, but resolved spontaneously by the second postoperative week without any sign of infection.
A decrease in graft volume was noticed in all animals in all five groups (Fig. 2).
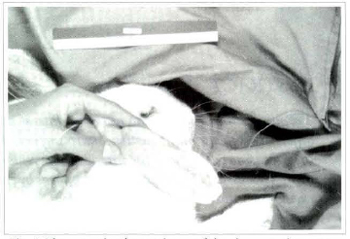
Fig. 2 Photograph of a spcecimen of the sharp suction group on the 360th postoperative day.
Descriptive Microscopy
On the 7th day
The specimens in all different groups were similar, but there was greater inflammatory reaction in those tissues harvesred by liposuction. Scar tissue surrounded the graft, and eventually penetrated into the spaces between fragments of the graft. inflammatory reaction consisted mainly of Lymphocytes and plasma cells, mostly distributed in the petiphery (Figs. 3 & 4). The central portion of transplant was densely populated by fat cells and their cellular architecture seemed to be preserved.
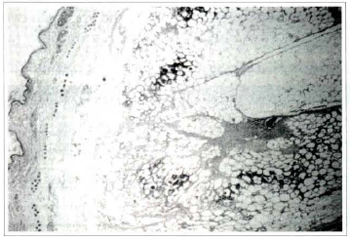
Fig. 3a - Photomicrography of fat tissue in the dissection group on rhe seventh postoperative day: ruptured adipocytes are evident at the periphery of this section with inflammatory cells permeating the area (Haematoxylin and eosin, original magnification x25).
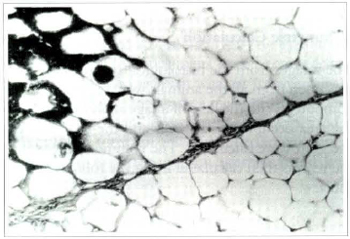
Fig. 3b - Photomicrography of fat tissue in the dissection group on the seventh postoperative day: detail of the same photomicrography showing some ruptured fat cells surrounded by polymorphonuclear leukocytes and others surrounded by macrophages (Haematoxylin and eosin, original magnification x160).
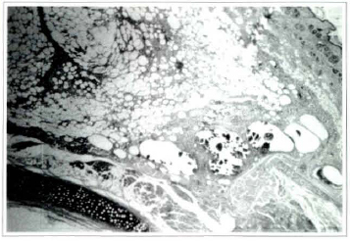
Fig. 4 . Photomicrography of fat tissue in the sharp suction group on the seventh postoperative day: ruptured adipocytes and different sizes of cysts can be seen (Haemacoxylin and eosin, original magnification x25).
On the 180th day
In all five groups the adipose tissue was casily identifiable and was surrounded by a discrete layer of connective tissue. The graft was still populated by many fat cells with preserved architecture. The inflammatory reaction persisted, but it consisted mainly of macrophages and giant cells containing large vacuoles filled with lipid. A stroma of connective tissue permeated the graft, tending to be more fibrous in the center. Additional cystic formations containing lipid, and rare areas of calcification were also observed within the tissue (Fig. 5).
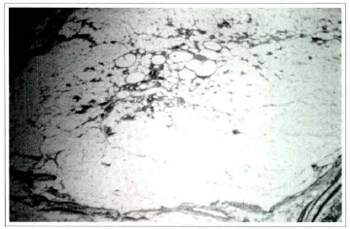
Fig. 5a - Photomicrography of fat tissue of the treatment group on the 180th postoperative day. The graft is surrounded by fibrous connective tissue that forms penetrating septa in some areas. There is inflammation in the center of the graft, (Haemaroxylin and eosin, original magnification x25).
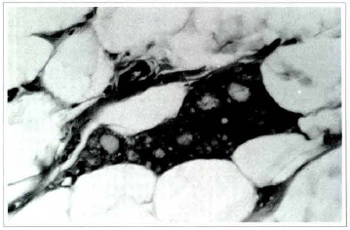
Fig. 5b - The same section in a bigger augmentation showing a giant cell with its vacuolized cytoplasm (Haematoxylin and eosin, x400).
On the 360th day
In most sections the grafted tissue was present, and well preserved fat cells predominated. The same type of inflammatory reaction observed at 180 days was also observed now, but it was much evener (Figs. 6 & 7).
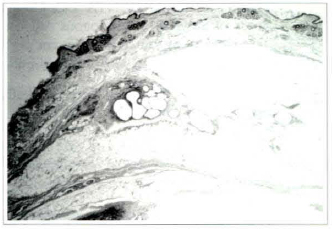
Fig. 6a - Photomicrography of fat tissue of the lavage group on the 360th postoperative day: graft, surrounded by thin layer of fibrous connective tissue (Haematoxylin and eosin, x25).
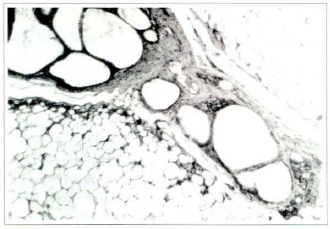
Fig. 6b . Photomicrography of fat tissue of the lavage group on the 360th postoperative day: large size cysts surrounded by repair tissue and phagocytic cells (Haemaroxylin and eosin, x1OO).
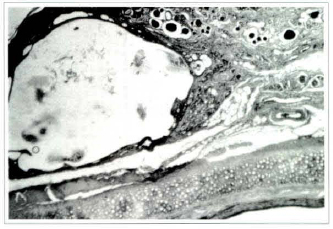
Fig. 7 - Photomicrography of fat tissue of the blunt suction group on the 360th postoperative day showing large cyst with cellular remains in its interior. Fat tissue has been almost totally substituted by fibrous connective tissue and phagocytic cells. Small areas of adipocytes within the connective tissue (Haemaroxylin and eosin, x25).
Morphometric Analysis
The samples of fat tissue that were used as controls showed an average percentage volume of 83% of fat cells and 17% of stroma. The calculated constant of tissue retraction (k) was 2.29.
Tables I, II, and III show the average percentage amount of each e1ement: fat cells, stroma, cystic formation, and inflammatory reaction assessed by counting at 7, 180, and 360 days, respectively. The inflammatory reaction decreased progressively, and was low by 360 days postoperatively.
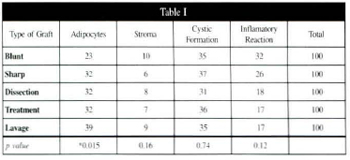
Table I - Average percentage amounts adipocytes, stroma, cystic formation, and inflammatory reaction assessed by counting on the seventh postoperative day, with correspoding p values.
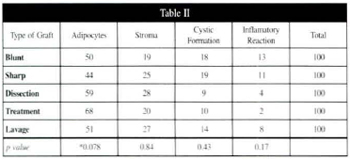
Table II - Average percentage amounts of adipocytes, stroma, cystic formation, and inflammatory reaction, with corresponding p values.
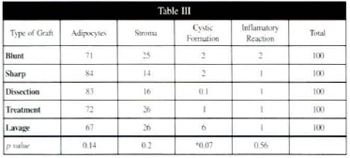
Table III - Average percentage amounts of adipocytes, stroma, cystic formation, and inflammatory reaction, assessed by counting on 360th postoperative day, with corresponding p values.
The average percentage volume of surviving grafted tissue in the five different groups is shown ar the three time intervals in table V. The maintenance of volume was similar between treatment groups, except at 180 days (p=0.0005). Table V shows the average percentage volume of surviving fat cells only. In the sharp suction and dissection groups the volume observed at seven days was maintained throughout the whole period of the study. Those same groups had the best survival rates.
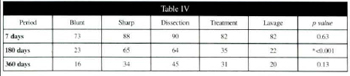
Table IV - Average percentage volume of surving grafted tissue in the five differente groups at 7, 180, and 360 days, with corresponding p values.
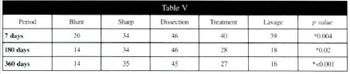
Table V - Average percentage volume of surving fat cells in the five different groups at 7, 180, and 360 days, with corresponding p values.
DISCUSSION
Histological evaluation of the whole specimen, as opposed to a sample biopsy only, was chosen on the assumption that it would result in a more accurate quantification of graft survival.
The wide variations found between sections of the same specimen and even within the same section, confirmed these assumptions.
The histopathological findings of this study were: fat cell degeneration, cystic formation, presence of lipid filled vacuous, formation of connective tissue capsules, and lymphocytic and plasmatic infiltration, are similar to those reported in previous studies of fat tissue grafts{1O,14,15,17,19). In the center of the specimen inflammatory cells and fibrous connective tissue had replaced fat cells, as described by GURNEY(10), and ROSSATTI(19).
The method of blunt suction resulted in the greatest degree of inf1ammatory reaction at all time and the lowest rare ofgraft, survival, while sharp suction produced the second highesr survival rate. These findings are possibly related to the injury caused to the tissue by the harvesting method, which used cannulas with blunt edged suction holes.
The assumption that adding culture medium would enhance fat cell survival was not confirmed by our findings; the final volume of these cells was smaller in the treated group than in the non-treated one harvested in the same way (Table V). Likewise washing with Ringer's lactate in the lavage group reduced graft survival, possibly as a result of the osmotic gradient on membrane integrity because the so called "physiological" solutions were originally formulated to be isotonic with red blood cells, and cell osmolarity may vary in other tissues. The fact that there was more cystic formation in the lavage group may strengthen that hypothesis, assuming that these cysts originated from cell degeneration. These findings do not support the recommendations of a number of authors(2, 3, 8, 9, 11, 12) that insist on thorough lavage of the adipose tissue before injection, or those of the American Society of Plastic and Reconstructive Surgeons(1) that also advocate the use of Ringer's lactate solution for the same purpose.
The high percentage volume of fat cells present at 360 days (67% to 84%), lead to the assumption that at this stage the graft is reaching a point of stability as the control rissue contained 83% of fat cells.
In the sharp suction group the maintenance of fat cell volume throughout the experiment, together with the low inflammatory reaction at 360 days, suggest that injury to the adipocytes occurred mainly at the time of harvesting, but the dissection group, which probably had the least injury during harvest, had a final volume of far cells of only 45%. Because the tissue in this group was immersed in saline while being fragmented into smaller pieces, the same phenomena of membrane rupture hypothesized in the lavage group could apply.
In the process of healing, as a result of the inflammatory reaction, toxic substances such as free radicals are produced. These substances enhance the injury to the surrounding tissues and promote resorption of the ten cells, whether or not they are transplanted. For this reason it is possible that the use of agents that neutralize free radicals may increase the overall survival of fat tissue grafts KONONAS et al.(13) compared fat grafts obtained by suction using blunt edged cannulas to those obtained by direct dissection. At nine months they found that the survival within the dissection group was 42% compared to that in the suction group, which was 32%.
Our findings were similar for direct dissection. The survival rate found by Kononas et al. in the blunt group is, however, higher than that tound in this study in the blunt suction group, and is consistent with thar of the sharp suction group. In this study, fat graft survival was assessed by volumetric analysis and an extensive qualitative evaluation, allowing a more precise interpretation of the results. To our knowledge such a thorough analysis has never been done before.
REFERENCES
1. AMERICAN SOCIETY OF PLASTIC AND RECONSTRUCTIVE SURGERY. Report on Aurologous fat Transplantation by the A.S.P.R.S.. Ad Hoc Committee on New Procedures. Chicago: A.S.P.R.S., 1987. 174p.
2. ASKEN S. FACIAL Limposuction and Microlipoinjection. J. Dermatol. Surg. Ocol. 1988 14:297-305.
3. ASKEN S. Limposuction Surgery and Autologous fat Transplantation. Connecticut: Appleton & lange, 1988. 166p.
4. BARTYNSKI J, MARION MS, WANGTD. Histopothologic Evolution of Adipose Autografts in a Rabbit Ear Model. Otolaryngol. Head Neck Surg. 1990; 102:314-321.
5. CURI M, SINGER MJM, IACONELLI LM, NACCACHE FA, ALONSO N, VIANNA MR. Transplante Autólogo de Gordura em Ratos. Rev. Paul. Med. 1991; 109:24-26.
6. EPPLEY BL, SMITH PG, SADOVE AM, DELFINO JJ. Exaperimental Effects of Graft, Reavascularization and Consistency on Cervicofacial Fat Transplant Survival. J. Oral. Maxillofac. Surg. 1990; 48:54-62.
7. ERSEK R. Transplantation of purified Autologous Fat: A 3-Year Followp is Disppointing. Plast. Reconstr. Surg. 1991; 87:219-228.
8. FOURNIER PE Harsard et Lipo-Extraction: Reflection et Lipoplastie. Rev. Chir. Esthet. Lang. Fr 1986;11:51-62.
9. FOURNIER PE Liposculpture: the Syringe Technique. Paris, Arnette Blackwell, 1991. 412p.
10. GURNEY CE. Experimental Study of the Behavior of Free Fat Transplants. Surgery 1938; 3:679-692.
11. HORL HW, FELLER AM, BIEMER E. Techinique for Liposuction Fat Reimplantation and Long-Term Volume Evaluation by Magnetic Resonance Imaging. Ann. Plat. Surg. 1991;26:248-258.
12. ILLOUZ YG. Reutilação do Tecido Adiposo Lipoaspirado. In: AVELAR JM, ILLOUZ YG. Lipoaspiração. São Paulo: Hipócrates, 1986. p. 117-122.
13. KONONAS TC, BUCKY LP, HURLEY C, MAY Jr JW. The Fate of Suctioned and Surgically Removed Fat after Reimplantation for the Soft Tissue Augmentation: A Volumetric and Histologic Study In The Rabbit. Plast. Reconstr. Surg. 1993; 91:763-768.
14. LEXER E. Zwanzig Jahre Transplantationsforshung in der Chirurgie. Arch. Clin. Chir. 1925; 138:251-302.
15. NEUHOF H. Brief Communication and Case Report: Free Transplantation of Fat for Bronchopulmonary Cavity. Ann. Surg. 1941; 113: 153- 155.
16. NGUYEN A, PASYK KA, BOUVIER TN, HASST CA, ARGENTA LC. Comparative Study of Survival of Autologous Adipose Tissue Taken and Tranplanted by Different Techniques. Plast. Reconstr. Surg. 1990; 85: 378-389.
17. PEER LA. The Neglected "Free Fat Graft, its Behavior and Clinical Use. Am. J. Surg. 1956; 92:40-47.
18. PEER LA. The Neglected "Free Fat Graft". Plast. Reconstr. Surg. 1956; 18:233-249.
19. ROSSATI B. Revascularization and Phagocytosis in Free Fat Autografts: an Experimental Study. Br. J. Plast. Surg. 1960; 13:35-41.
20. SAMDAL F, SKOLLEBORG KC, BERTHELSEN B. The Effect of Preoperative Needle Abrasion of the Recipient Site on Survival of Autologous Free Fat Grafts in Rats. Scand. J. Plast. Reconstr. Hand Surg. 1992; 26:33-36.
I - Associated Professor, Plastic Surgery division, Universidade Federal de São Paulo (EPM). Associated Professor, Plastic Surgery and Burns division, Universidade Federal de São Paulo.
II - Postgraduated in Surgical Clinic of the Faculdade de Medicina da Universidade de São Paulo.
III - Associated Professor and chief of Laboratory of Experimental Atmospheric Polution of Faculdade de Medicina da Universidade de São Paulo.
IV - Research Fellow in Plastic Surgery, Medical College of Wisconsin, USA.
V - Head Professor of the Plastic Surgery and Burns Discipline of the faculdade de Medicina da Universidade de São Paulo.
Address for Correspondence:
Americo Marques, MD
Av. Republica do Líbano, 894
04502-001 - São Paulo - SP - Brazil


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter