

Case Report - Year 2021 - Volume 36 -
Kaposiform hemangioendothelioma: radiological, surgical and anatomopathological correlation
Hemangioendotelioma kaposiforme: correlação radiológica, cirúrgica e anatomopatológica
ABSTRACT
Introduction: Kaposiform cutaneous hemangioendothelioma (HEK) is a rare locally aggressive vascular tumor, seen mainly in newborns and children. It has a prevalence of 0.91 cases per 100,000 children, being most common in the extremities. The treatment of choice is total resection; however, it is often not possible due to the lesion's extent and association with the Kasabach-Merritt phenomenon.
Objectives: To describe the evolution of a rare tumor in the plantar region of a child, correlating the radiological, surgical, and histopathological findings.
Methods: The authors report the case of a boy admitted at the age of five with a recurrent painful plantar skin lesion. In the magnetic resonance examination (NMR), he presented a lesion in the posterior plantar region measuring 3cmx2cm, superficial to the plantar fascia. In the biopsy examination, he revealed kaposiform hemangioendothelioma without association with the Kasabach-Merritt phenomenon. He underwent a surgical procedure for excision and presented recurrence after six months. A new broad resection, reconstruction with a plantar flap, and partial skin graft were performed, obtaining free margins, with no recurrence in the 15-year follow-up.
Conclusion: Clinical findings suggested plantar fibromatosis, NMR helped in delimiting the tumor, and histopathological examination with immunohistochemistry confirmed the diagnosis of kaposiform cutaneous hemangioendothelioma. Resection was performed up to the fascia with recurrence, requiring re-approach and resection to the periosteum with reconstruction using a plantar flap and skin graft, without recurrence after 15 years. The authors call attention to the wide resection of deep and lateral margins to control tumor growth.
Keywords: Hemangioendothelioma; Hemangioma; Plastic surgery; Surgical pathology; Neoplasms of vascular tissue
RESUMO
Introdução: O hemangioendotelioma cutâneo kaposiforme (HEK) é um tumor vascular raro localmente agressivo, visto principalmente em recém-nascidos e crianças. Tem prevalência de 0,91 casos por 100.000 crianças, mais comum nas extremidades. O tratamento de escolha é a ressecção total, todavia muitas vezes não é possível devido à extensão da lesão e associação ao fenômeno de Kasabach-Merritt.
Objetivos: Descrever a evolução de caso raro de tumor na região plantar de criança, correlacionando os achados radiológicos, cirúrgicos e histopatológicos.
Métodos: Os autores relatam o caso de menino admitido aos cinco anos de idade com lesão cutânea plantar dolorosa recidivada. No exame de ressonância magnética (RMN) apresentava lesão na região plantar posterior medindo 3cmx2cm, superficial à fáscia plantar, no exame de biópsia revelou hemangioendotelioma kaposiforme, sem associação com o fenômeno de Kasabach-Merritt. Foi submetido a procedimento cirúrgico para exérese, apresentou recidiva após seis meses. Foi realizada nova ressecção ampla, reconstrução com retalho plantar e enxerto de pele parcial, obtendo-se margens livres, sem recidiva no seguimento de 15 anos.
Conclusão: Os achados clínicos sugeriam fibromatose plantar, a RMN auxiliou na delimitação do tumor, o exame histopatológico com imunohistoquímica confirmaram o diagnóstico de hemangioendotelioma cutâneo kaposiforme. Realizou-se ressecção até a fáscia com recidiva, sendo necessária reabordagem e ressecção até o periósteo com a reconstrução com retalho plantar e enxerto de pele, sem recidiva no seguimento de 15 anos. Os autores chamam a atenção para a ressecção ampla de margens profundas e laterais para controle do crescimento tumoral.
Palavras-chave: Hemangioendotelioma; Hemangioma; Cirurgia plástica; Patologia cirúrgica; Neoplasias de tecido vascular
INTRODUCTION
Kaposiform hemangioendothelioma (KHE) is a rare vascular tumor that comprises a wide range of life-threatening superficial or infiltrative lesions. It has a prevalence of 0.91 cases per 100,000 children, being most common in the extremities. When it appears in the elderly, it has a greater malignancy chance and may develop angiosarcoma or hemangiosarcoma. When it occurs in children, it is benign1-3. The other histological differential diagnoses of KHE include infantile hemangioma, congenital hemangioma, fusocellular hemangioma, verrucous malformation/hemangioma and Kaposi’s sarcoma1.
The microscopy examination is characterized by confluent nodules of spindle-shaped neoplastic endothelial cells involving multiple planes of tissue that are positive for endothelial, lymphatic, and smooth muscle markers2. The treatment approach for these tumors is variable, mainly because the cases are rare, can be infiltrative and located in an area of difficult surgical resection, and can also be related to the Kasabach-Merritt phenomenon, characterized by an association of capillary hemangioma and thrombocytopenia, which can cause bleeding, petechiae, bruises, and spontaneous bruising4,5. Spontaneous tumor involution is rare; the best treatment option is total excision. However, it is sometimes mutilating or unviable, and other treatment options, use of corticosteroids, vincristine, interferon, chemotherapy, embolization, propanolol, sclerotherapy, and radiotherapy have been described2.
This article aims to describe the radiological correlation using resonance, surgical and anatomopathological findings in a rare case of a child with kaposiform hemangioendothelioma.
CASE REPORT
Boy admitted at the age of five in the Orthopedics Service of Hospital Sarah, presenting a history of onset, at six months of age, of a tumor in the right plantar region, painless, with wine aspect without inflammatory signs. He underwent two surgical procedures for excision in another medical service, at the age of two, with recurrence after three months, and at the age of four, with recurrence afterward, having been diagnosed with plantar fibromatosis. When he was admitted to this hospital, he had tumor recurrence in the plantar region and difficulty to perform plantar support on the right and to wear shoes, due to local pain. He presented a hardened tumor with a scar aspect on physical examination, not painful on palpation, with an extension of about 5 cm in its largest diameter, located in the right plantar region (Figure 1). AP and lateral plantar radiography and nuclear magnetic resonance imaging showed a lesion in the posterior plantar region measuring in the lateral-lateral direction, 3cm long x 2cm thick, respecting the plantar fascia and directed to the skin. He had a normal blood count, platelet count, and coagulogram, with no Kasabach-Merritt phenomenon. A biopsy was indicated, whose report showed kaposiform cutaneous hemangioendothelioma. Marginal tumor excision was performed, maintaining the fascia and primary suture of the lesion measuring 3x2cm; he had a recurrence six months after the operation. It was decided to enlarge the lateral surgical margin, and in-depth by 2 cm, elliptical resection was performed, including skin, subcutaneous, muscular fascia up to the calcaneus, measuring 5x3x2cm.
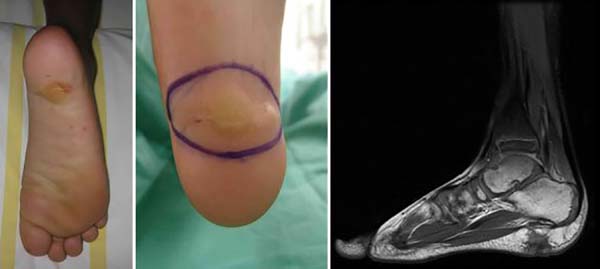
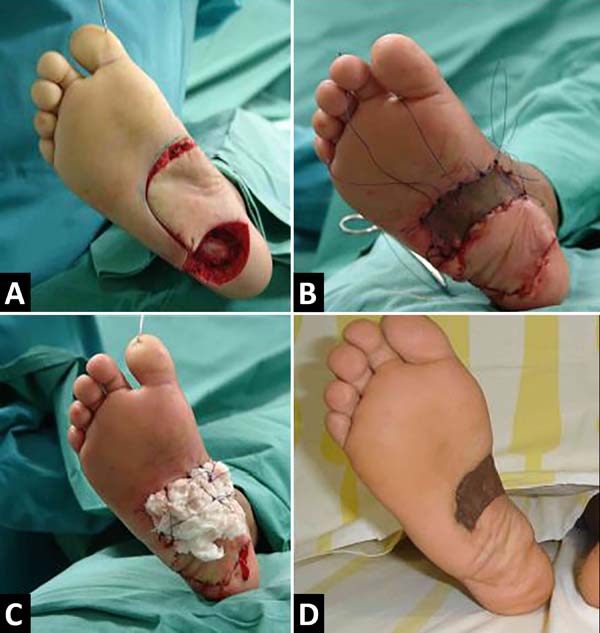
The intraoperative freezing exam showed fibrotic tissue, but with no evidence of a tumor in the deep margin, with the excision of the plantar fascia, the calcaneus periosteum, and reconstruction with flap rotation of the medial pedicle plantar cavity measuring 6x4cm and skin grafting partial in the donor region in the midfoot, 4-0 monocryl suture and Brown dressing in the grafted area. The graft was removed with Blair’s knife from the medial portion of the homolateral thigh; the dressing was maintained for a week when total skin graft integration was observed. Rest with an elevated limb without load was instructed until complete flap and skin graft integration for two weeks; the flap’s satisfactory evolution with remission of pain was observed, without tumor recurrence in the 15-year follow-up (Figure 2).
The anatomopathological examination revealed vascular neoplasia with infiltrative lobes, separated by fibrous septa, containing compact, sometimes loose, fusocellular areas, in capillary permeate, cracks, and vessels varying sizes, covered by endothelium with a glomeruloid aspect, few figures of mitosis and free surgical margins. Tumor karyotype (45, XY, -10 (3) / 46, XY (21) with chromosome 10 monosomy (3 cells), normal clone (21 cells) culture time 8 days.
DISCUSSION
The natural history of kaposiform hemangioendothelioma, the differential diagnosis, and treatment regime for these lesions remains unclear and challenges plastic surgeons’ challenges. The authors describe the report of a rare case of kaposiform hemangioendothelioma with a difficult diagnosis, which had previously been operated on and relapsed three times. He presented a fibromatous aspect on physical examination, leading to the clinical suspicion of plantar fibromatosis, which was not confirmed by imaging and biopsy exams. In a biopsy, histopathological findings, corroborating with the literature, were of confluent nodules of spindle-shaped neoplastic endothelial cells involving multiple tissue planes that were positive for smooth endothelial, lymphatic, and muscle markers; this was important for the definition of the diagnosis.
In the first surgery for excision, the oncological criteria were respected, preserving the fascia, following the NMR exam, which showed involvement in the deep dermis, deep, subcutaneous, and muscular tissues; however, these parameters were not sufficient for remission. of the tumor. After six months postoperatively, there was a recurrence, and a new surgical approach was performed, widening the lateral and deep margins, with the excision of the fascia and periosteum, achieving remission during 15 years of follow-up without important functional sequelae.
Kaposiform hemangioendothelioma tumor is a tumor of vascular origin according to the classification of the International Society for the Study of Vascular Anomalies (ISSVA), which covers all malformations and vascular tumors in an internationally consistent nomenclature framework. It is one of two systems of most used classification. It is based on Mulliken and Glowacki’s initial classification in 19826 and has since been updated with the recognition of causal genetic mutations; this classification was revised in May 2018. Excision with a tumor-free surgical margin should be the definitive treatment for KHE, although it is unattainable in some cases due to the location, extent of the lesion, or association with coagulopathy. When associated with coagulopathy, it can have a mortality rate of around 20%. Thus, other treatment options are described, such as the use of corticosteroids, single or combined chemotherapy, laser, and radiotherapy5-8.
Sirolimus®, also called rapamycin, is a drug used as an immunosuppressant. It is a macrocyclic lactone produced by organisms of the species Streptomyces hygroscopicus, a macrolide mTOR inhibitor with an antiangiogenic effect. It has been reported as a successful agent in treating refractory and complicated cases of KHE, reducing tumor, pain, and coagulopathy phenomena. However, there is no single defined protocol, and its use in children with vascular anomalies is limited. It was usually indicated when other alternatives failed.
The reconstruction of the plantar region, corroborating with other authors9-12 and as presented in the case report, was a challenge due to the need to have the flap with sufficient dimensions to cover the defect and protect the calcaneus, to have skin similar to the region, low volume and sufficient sensitivity to prevent injuries, support load, avoid pain when walking and enable the use of shoes. Among the available options are the rotating plantar cavity’s local flaps, the medial plantar flap, the reverse sural flap, distance flaps, and free flaps9-12. It was not possible to perform a skin graft due to bone exposure. In this case report, it was possible to cover a 5 cm defect in the largest axis located in the calcaneus region, using the plantar cavity flap and partial skin grafting in the donor area. The flap and skin graft showed good integration, which allowed for the functional recovery of the foot.
CONCLUSION
In this report of the case of a child with kaposiform hemangioendothelioma, a rare vascular tumor, it was not possible to make a clinical diagnosis at admission. A biopsy and histopathological examination were necessary. In the NMR exam, diffuse heterogeneous enhancement was identified after contrast of the lesion involving the skin, from the subcutaneous tissue to the fascia. These limits were used for resection up to the fascia, but they were not sufficient for tumor control, and there was recurrence after six months. It was necessary to re-approach the resection of the fascia and periosteum to control the vascular lesion’s growth, which, although benign, has endothelial growth with an invasion of the skin, muscle, fascia to the periosteum. The exeresis was performed on the lateral margins and in-depth, up to the periosteum, and extensive reconstruction with the plantar cavity flap and partial skin grafting in the donor region, with no signs of recurrence after 15 years. We emphasize the surgeon’s importance to go beyond the margins delimited by NMR for the control of the disease.
REFERENCES
1. Putra J, Gupta A. Kaposiform haemangioendothelioma: a review with emphasis on histological differential diagnosis. Pathology. 2017 Jun;49(4):356-62.
2. Rodriguez NZ, Benevides PJ. Sirolimus (rapamicina) en pacientes con hemangioendotelioma kaposiforme: caso clínico. Rev Chil Pediatr. 2013 Out;84(5):537-44.
3. International Society for the Study of Vascular Anomalies (ISVVA). Classificação de malformações e tumores vasculares [Internet]. Melbourne: ISVVA; 2018; [acesso em 2019 Jun 27]. Disponível em: http://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf
4. Mukai AO, Zanlochi AGS, Elias CPF, Rolim CH, Baida LL, Tutia PC, et al. Hemangioendotelioma kaposiforme e síndrome de Kasabach-Merritt. Rev Paul Pediatr. 2008 Jun;26(2):192-6.
5. Hiraki PY, Goldenberg DC. Diagnóstico e tratamento do hemangioma infantil. Rev Bras Cir Plást. 2010;25(2):388-97.
6. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast. Reconst. Surg. 1982; 69(3):412-22
7. Pope E, Krafchik BR, Macarthur C, Stempak D, Stephens D, Weinstein M, et al. Oral versus high-dose pulse corticosteroids for problematic infantile hemangiomas: a randomized, controlloed trial. Pediatrics. 2007 Jun;119(6):e1239-47.
8. Léauté-Labrèze C, Taïeb A. Effecacité des bêtabloquants dans les hémangiomes capillaires infantiles: signification physiopathologique et conséquences thérapeutiques. Ann Dermatol Venereol. 2008 Dez;135(12):860-2.
9. Martins GB, Moreira AA, Viana FO. Reconstrução de lesões de partes moles do calcanhar com o uso de retalhos fasciocutâneos. Rev Bras Cir Plást. 2009;24(1):104-9.
10. Barreiro GC, Baptista RR, Busnardo F, Olivan M, Ferreira MC. Reconstrução de planta de pé de acordo com o conceito das subunidades anatômicas. Rev Bras Cir Plást. 2010;25(3):81.
11. Benito-Ruiz J, Yoon T, Guisantes-Pintos E, Monner J, Serra-Renom JM. Reconstruction of soft-tissue defects of the heel with local fasciocutaneous flaps. Ann Plast Surg. 2004 Abr;52(4):380-4.
12. Garcia AMC. Retalho sural reverso para reconstrução distal da perna, tornozelo, calcanhar e do pé. Rev Bras Cir Plást. 2009;24(1):96-103.
1. Rede Sarah de Hospitais, Plastic Surgery,
titular member of SBCP, Brasília, DF, Brazil.
2. Rede Sarah de Hospitais, Nursing, Brasília, DF,
Brazil.
3. UNICEUB, Medical Student, Brasília, DF,
Brazil.
4. Fundação de Ensino e Pesquisa em Ciências da
Saúde Escola Superior de Ciências da Saúde, Nursing, Brasília, DF,
Brazil.
KTB Investigation, Methodology, Realization of operations and/or trials, Writing - Original Draft Preparation
HJA Realization of operations and/or trials
MIZG Analysis and/or data interpretation, Supervision
SYM Final manuscript approval, Project Administration
*Corresponding author: Maria Ireni Zapalowski Galvão SMHS Quadra 501, Bloco A, Asa Sul, DF, Brazil. Zip Code: 70335-970 E-mail: 201995@sarah.br
Article received: August 06, 2019.
Article accepted: January 10, 2021.
Conflicts of interest: none



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter