

Ideas and Innovation - Year 2020 - Volume 35 -
Evaluation of the effectiveness of a modified liposuction protective device: an experimental study in swine
Avaliação da eficácia de dispositivo protetor de lipoaspiração modificado: estudo experimental em suínos
ABSTRACT
Liposuction is one of the most common procedures in the plastic surgery specialty. In the 2018 ISAPS survey, it was the second most performed surgery worldwide. Due to the repetitive movements typical of the surgery, significant friction is generated at the site, and the consequences are skin burns that can leave unsightly scars and dyschromias. This study aims to create a skin protective device prototype from an old model, which serves this purpose, and to observe its functionality and its effects on the pigskin. The tests were carried out on dead animals without suffering provided by the Veterinary Medicine sector at Universidade Positivo. Three incisions were made in the animal's abdomen to pass the liposuction cannula and another three for the insertion and use of the prototype to compare it with the model device. The established time for liposuction movements was twenty minutes, performed with the 5mm cannula directly in contact with the skin and inside the prototype. The prototype's ergonomics, ease of insertion, and good locking on the skin with different tractions were evaluated. Observation and evaluation of the skin were performed after procedures and incisions' measurements (cm). The cutaneous liposuction protective device prototype presented easy handling and a more efficient skin locking mechanism than the model used. The skin incision for using the prototype was slightly larger, and the skin showed no burning signs.
Keywords: Lipectomy; Plastic surgery; Hypertrophic scar; Scar; Burns; Comparative study; Innovation.
RESUMO
A lipoaspiração é um dos procedimentos mais comuns na especialidade de cirurgia plástica. No levantamento da ISAPS de 2018 foi a segunda cirurgia mais realizada em todo o mundo. Devido aos movimentos repetitivos próprios da cirurgia, fricção importante é gerada no local e as consequências são queimaduras cutâneas que podem deixar cicatrizes inestéticas e discromias. O objetivo deste estudo é criar um protótipo de um dispositivo protetor da pele, a partir de um modelo antigo, que sirva a esse propósito, e observar sua funcionalidade e os efeitos da sua utilização na pele de suínos. Os testes foram realizados em animais mortos sem sofrimento disponibilizados pelo setor de Medicina Veterinária da Universidade Positivo. Foram feitas três incisões no abdome do animal para passagem da cânula de lipoaspiração e outras três para a inserção e utilização do protótipo, bem como para comparação com o dispositivo modelo. O tempo estabelecido de movimentos de lipoaspiração foi de vinte minutos, realizados com a cânula de 5mm diretamente em contato com a pele e dentro do protótipo. Foi avaliada a ergonomia do protótipo, facilidade de inserção e travamento adequado na pele com diferentes trações. Observação e avaliação da pele após os procedimentos e medição (cm) das incisões foram realizadas. O protótipo do dispositivo protetor cutâneo de lipoaspiração criado apresentou fácil manuseio e mecanismo de travamento na pele mais eficiente quando comparado ao modelo utilizado. A incisão cutânea para uso do protótipo foi ligeiramente maior e a pele não apresentou sinais de queimadura.
Palavras-chave: Lipectomia; Cirurgia plástica; Cicatriz hipertrófica; Cicatriz; Queimaduras; Estudo comparativo; Inovação
INTRODUCTION
Liposuction is one of the most common procedures in plastic surgery specialty1,2. In the 2018 ISAPS survey, it was the second most performed surgery worldwide, after breast augmentation, with Brazil only behind the USA in the period in the number of liposuction3.
Surgery can be done with the traditional technique (vacuum), vibroliposuction, ultrasonic, and laser lipolysis. The incisions are usually 3 to 7mm. Due to the repeated movements of coming and going necessary for uniform fat removal, carried out continuously for minutes or hours, significant friction is generated on the spot, and the consequences are skin burns that can leave unsightly scars and dyschromias1,2,4,5.
In general, the most severe skin burns occur when the ultrasonic technique is used, whose energy generated by the ultrasound waves raises the cannula’s temperature and the site a lot. In this technique, skin protection is mandatory4,5. However, other techniques, such as vibroliposuction6 or traditional liposuction, can also cause skin burns.
The use of a protector (device) that creates a physical barrier between the skin and the liposuction cannula is desirable to improve the results, especially in ultrasonic liposuction.
OBJECTIVE
This study aims to create a skin protective device prototype from a model that serves this purpose and to observe its functionality and its effects on the pigskin.
METHODS
This is an experimental study, approved by the Research Ethics Committee on the Use of Animals of the Centro de Estudos Superiores Positivo Ltda. under protocol 445 and opinion 3424
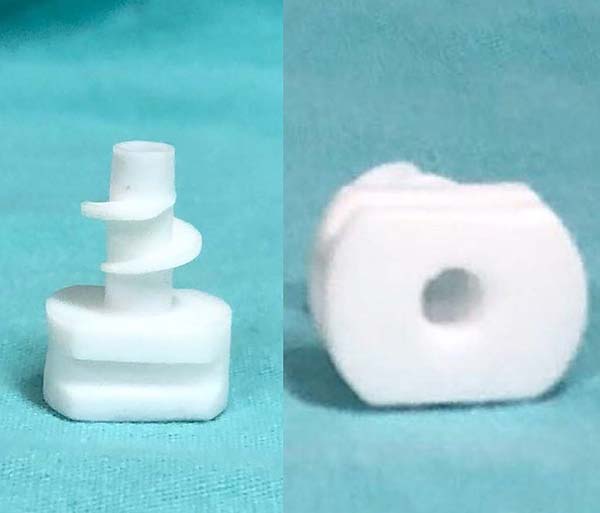
An end-of-life device (Figure 1), produced by a Brazilian company (Industra Technologies, São Carlos, Brazil), was used as a reference. It was made in injectable polypropylene with molds for the injection of the material. This process requires large-scale production to justify costs. Its dimensions are approximately 2.5 cm high and 2 cm wide, with grooves that make it difficult to clean and with a screw mechanism for insertion/locking. The insertion is made through a cutaneous incision of approximately 1 cm with a Kelly clamp since manual insertion is difficult due to the reduced size of the device’s base. Besides, the need to change the Kelly clamp’s side at each turn of the thread of the device generates an increase in the insertion and removal time.
Prototypes were designed using SolidWorks 3D CAD software (Dassault Systèmes SolidWorks Corporation, MA, USA) and printed on a MakerBot Replicator + 3D printer (MakerBot Industries, NY, USA) using lactic polyacid (PLA) as the printing material (Figure 2). The design of our own creation, is 1.9 cm high and 2.5 cm wide. The prototype base is rounded and with shallow grooves to facilitate manual locking. The thread, with a less spaced spiral and with a larger diameter, aims to facilitate its insertion while increasing its potential to lock into the skin (Figure 2). The difference between the screw mechanism of the prototype and the model device is evident in the side view (Figure 3).
Animal tests were carried out on two pigs provided by the Veterinary Medicine sector at Universidade Positivo (Curitiba/PR). One of the authors conducted the procedures in the vivarium’s operating room, with the animals submitted to assisted painless death just before the procedure.
Six incisions were made in each animal’s abdomen, three for the passage of the 5mm liposuction cannula without protection, and another three for the comparative tests of insertion and locking between the prototype and the model device and, then, liposuction simulation using the prototype.
For the comparison, it was established a period of liposuction movements of twenty minutes performed with the 5mm cannula directly in contact with the skin and inside the prototype. Observation and evaluation of the skin were performed after the incisions’ procedures and measurements (cm).
The prototype’s ergonomics, ease of insertion, adequate locking on the skin with different tractions (light and intense), the skin’s appearance after 20 minutes of liposuction simulation, and the measurement of the incision size after the simulation were evaluated.
RESULTS
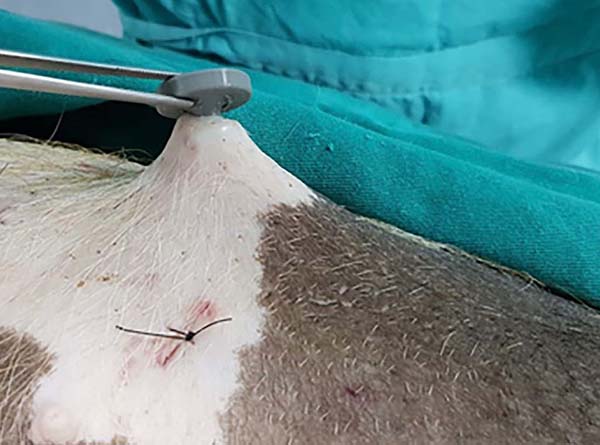
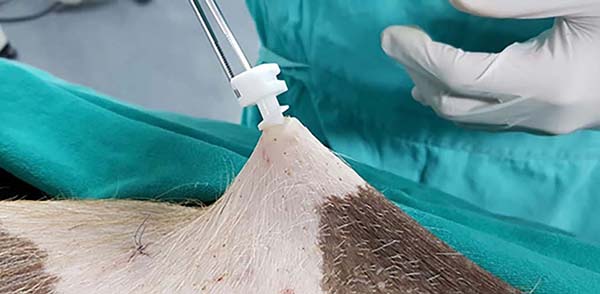
The prototype showed good ergonomics and cannula’s internal movement (Figure 4), but with slight resistance due to the 3D printing material. The insertion could be performed directly with the hand, without using the Kelly clamp as in the model, mainly due to the rounded shape and larger size of the device’s base and its lateral grooves (Figures 2, 3 and 5).
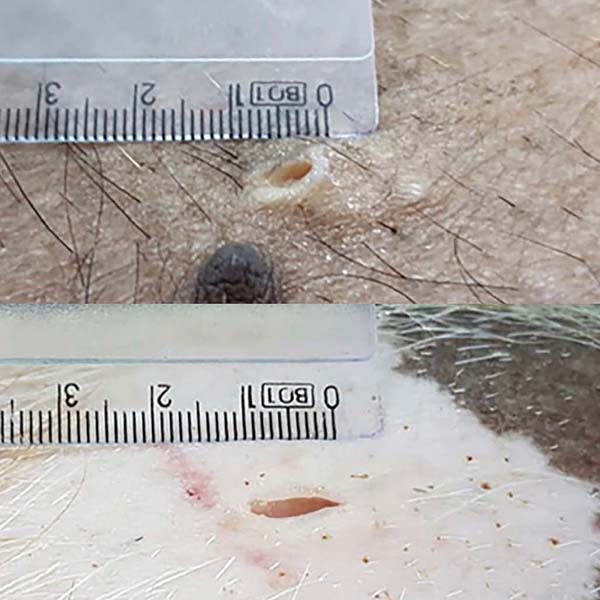
Both devices had good locking in the light traction test, but in intense traction, the old model device started to detach from the skin since the prototype maintained the locking (Figures 6 and 7). Only in an incision of an animal did the old device remain locked under intense tension. The skin had an adjacent burn, edema, and serous secretion in the incision after 20 minutes of liposuction simulation without the device. However, when using the device, the skin remained intact and unchanged. The minimum incision for the prototype’s insertion (10mm) was slightly larger than the conventional incision (6mm). In two incisions, one in each animal, the prototype incision size was eight and nine millimeters (Figure 8). Both the appearance and the average size were very similar in the other incisions.
DISCUSSION
The increase in demand for plastic surgery worldwide and Brazil is a proven reality in the latest ISAPS surveys, with liposuction being one of the champions in this regard3. This increase is also accompanied by patients’ demand for less expensive surgical procedures, faster recovery, and, obviously, better results. Details such as the scars resulting from liposuction, even if small and well-positioned in barely visible regions, can be a reason for complaints. The job market in this specialty is extremely competitive, so all details must be valued.
There are few studies in the literature with devices to protect the skin from the friction generated by the liposuction cannula, such as using a 1mL syringe section in the skin incision(7-8 )or a 6.5mm diameter nasopharyngeal cannula section as a resource protector9. However, in addition to these methods requiring suture points for fixing the syringe/cannula to the skin, which can generate unwanted scarring and increase surgical time, these studies do not present data on the healing of the incision or preclinical studies, nor a series of clinical cases. They only describe the method. Thus, the study we conducted is perhaps the first in this sense with experimental evaluation.
The fact that the prototype can be produced with 3D printers opens up a range of material options such as thermoplastics (PLA, ABS, PET, PEEK, and polyamide), metals (steel, titanium, aluminum, chrome-cobalt, nickel, bronze, and copper), resins (SLA and PolyJet)10. This method also allows production on demand, reducing time, operational and manufacturing costs, and provides adjustments and improvements via software at any time.
On the other hand, only some of these materials can be used in surgical practice due to exposure to autoclave temperature (polyamide and PEEK).
Metallic materials should be avoided because they are great conductors of mechanical and thermal energy, considerably increasing the risks of skin burns, which is precisely what we want to avoid.
The 3D printing of the prototypes, performed with PLA thermoplastic due to their ease of manipulation and experimentation, left small imperfections and material debris in the device, increasing the material’s friction with the cannula and the difficulty in insertion. However, such printing imperfections can be solved in the next prototypes by fine-tuning the printer’s parameters or using another material in the final product or by another manufacturing method such as injectors with prior prototyping. The latter would also allow for larger-scale production.
The choice of material is essential since it must withstand high temperatures, be non-toxic, have low friction, allow free movement of the cannula, and have a competitive cost. For these reasons, printing with thermoplastic polyamide (nylon) and PEEK (polyether-ether-ketone) can be used, but more experiments and studies need to be carried out to define the ideal material.
This study demonstrated an improvement in the prototype’s performance concerning the model device: better ergonomics for handling, the possibility of manual insertion without clamps, and superior locking without the need for fixation points. However, there was no clinical difference in cutaneous effects between them, not even in the size of the incision required for insertion (10mm).
The few studies available for comparison between skin protective devices for liposuction show only case reports, with no preclinical, experimental studies or even randomized clinical studies and with a significant “n.” The next step should be defining the best material to be used and a clinical study with the patient as its own control, that is, using the protective devices in some incisions and comparing it with the incisions where it was not used and also with the device model.
Limitations
One of the most significant limitations of this study is the small number of animals and incisions tested; however, we had to use what was offered to us by the vivarium and the time offered to perform it. The definition of the ideal material to be used in humans should undergo toxicity assessments, ease of production, sterilization tests, and costs to be studied in more depth.
CONCLUSION
The cutaneous liposuction protective device prototype presented easy handling and a more efficient locking mechanism on the skin compared to the model used, without the need for fixation as found in the literature.
The skin incision for using the prototype was slightly larger than without it, but the same as the model. The skin showed no signs of friction burn with the use of the prototype.
Clinical studies with a significant “n” should be performed to assess long-term healing, and further functionality studies are needed to define the appropriate material for making this device.
REFERENCES
1. Iverson RE & Pao VS. Liposuction: MOC-PSsm CME. Plast Recons Surg. 2008;121(4):1-11.
2. Matarasso A & Levine SM. Evidence Based Medicine: Liposuction. Plast Recons Surg. 2013;132(6):1697-705.
3. ISAPS. Global Statistics: Full Global Survey Results. 2018 [acesso 05 de março de 2020]. Disponível em: https://www.isaps.org/medical-professionals/isaps-global-statist
4. Graf R, Auersvald A, Damasio RC, Rippel R, Araujo LR, Bigarelli LH, Franck CL. Ultrasound-assisted liposuction: an analysis of 348 cases. Aesthetic Plast Surg. 2003;27(2):146-53.
5. Ablaza VJ, et al. Tissue temperatures during ultrasound-assisted liposuction. Plast Recons Surg. 1998;102(2):534-42.
6. Fodor PB, Vogt PA. Power-assisted Lipoplasty(PAL): A Clinical Pilot Study Comparing PAL to Traditional Liposuction (TL). Aesthetic Plast Surg. 21(1):90-2.
7. Bang J & Song DH. Inexpensive Method of Liposuction Cannula Port-Site Protection, Aesth Plast Surg. 2013;37:843.
8. Khoo LS, Corona GG, Radwanski H, Fernandes VS, Pintanguy IH. Inexpensive Method of Liposuction Cannula Port-Site Protection, Aesth Plast Surg. 2014;38:256-7.
9. Salhi S, Meunier F, Cordoba C. Use of a nasopharyngeal cannula as a skin protector during lipoaspiration. JPRAS Open. 2017;12:87-90.
10. Ngo TD, Kashani A, Imbalzano G, Nguyen KTQ, Hui D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Composites Part B: Engineering. 2018;143:172-96.
1. University Positivo, Department of Graduate Studies in Minimally Invasive Surgery
Curitiba, PR, Brazil.
Institution: University Positivo, Department of Graduate Studies in Minimally Invasive Surgery Curitiba, PR, Brazil.
LRRA Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Methodology, Project Administration, Realization of operations and/or trials, Writing - Original Draft Preparation, Writing - Review & Editing
MPL Final manuscript approval, Project Administration, Resources, Supervision
BH Analysis and/or data interpretation, Data Curation, Investigation, Methodology, Writing - Original Draft Preparation, Writing - Review & Editing
GSC Data Curation, Realization of operations and/or trials, Writing - Original Draft Preparation
LMNS Data Curation, Realization of operations and/or trials
MHBS Data Curation, Realization of operations and/or trials, Writing - Original Draft Preparation
Corresponding author: Luiz Roberto Reis Araújo, Alameda Presidente Taunay, 1820, Mercês, Curitiba, PR, Brazil. Zip Code: 80430-042. E-mail: drluiz@drluizaraujo.com.br
Article received: April 19, 2019.
Article accepted: October 05, 2020.
Conflicts of interest: none














 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter