

Original Article - Year 2020 - Volume 35 -
En bloc explant of silicone breast prostheses and quality of life and evolution of ASIA syndrome symptoms
O explante em bloco de prótese mamária de silicone na qualidade de vida e evolução dos sintomas da síndrome ASIA
ABSTRACT
Introduction: ASIA syndrome is the abbreviation for the
adjuvant-induced autoimmune syndrome, a syndrome that
encompasses autoimmune diseases triggered by silicone
and other substances. Evidence shows the association of
silicone breast implant with ASIA syndrome. The silicone
prosthesis explant is an essential resource in treating ASIA
syndrome; however, there are few studies analyzing the
improvement in symptoms and the quality of life that surgery
can provide.
Methods: Fifteen patients with ASIA syndrome
who underwent breast implant prosthesis and reconstruction
with mastopexy were analyzed. During the 12-month followup,
they were assessed for symptom evolution and, through
the Breast-Q® questionnaire, for quality of life.
Results: The most common symptoms, such as myalgia, arthralgia,
chronic fatigue, dry skin, and hair, were improved in more
than 80% of patients operated on after 12 months of followup.
There was an improvement in the quality of life after
the surgical procedure when compared to the preoperative.
Conclusion: Breast prosthesis explants in patients with
ASIA syndrome seem to be associated with improved quality
of life and decreased symptoms related to the syndrome.
Keywords: Autoimmune diseases; Breast diseases; Mammoplasty; Breast implant; Quality of life; Outcome measures reported by the patient.
RESUMO
Introdução: A síndrome ASIA é a abreviação em inglês de síndrome autoimune induzida por adjuvantes, síndrome que engloba doenças autoimunes desencadeadas por silicone e outras substâncias. Evidências mostram a associação da inclusão de prótese de mama de silicone com a síndrome ASIA. O explante de prótese de silicone é um recurso importante no tratamento da síndrome ASIA, porém há poucos estudos analisando a melhora dos sintomas e da qualidade de vida que a cirurgia pode proporcionar.
Métodos: Foram analisadas 15 pacientes com síndrome ASIA submetidas à explante da prótese de mama e reconstrução com mastopexia. Durante o acompanhamento de 12 meses foram avaliadas quanto à evolução dos sintomas e, através do questionário Breast- Q®, da qualidade de vida.
Resultados: Os sintomas mais comuns como mialgia, artralgia, fadiga crônica, pele e cabelos secos tiveram melhora em mais de 80% das pacientes operadas ao final de 12 meses de acompanhamento. Houve melhora na qualidade de vida após o procedimento cirúrgico quando comparado com o pré-operatório.
Conclusão: O explante de prótese de mama em pacientes com síndrome ASIA parece estar associado à melhora da qualidade de vida e diminuição dos sintomas relacionados à síndrome.
Palavras-chave: Doenças autoimunes; Doenças mamárias; Mamoplastia; Implante mamário; Qualidade de vida; Medidas de resultados relatados pelo paciente
INTRODUCTION
ASIA syndrome is the abbreviation for the adjuvant-induced autoimmune syndrome. Adjuvants are all materials foreign to the body that can trigger a chronic inflammatory process. Described in 2011 by the Israeli doctor Yehuda Shoenfeld, the syndrome encompasses autoimmune diseases that present similar symptoms triggered by adjuvants1.
The diseases described and their triggering agents are siliconomas (silicone), macrophage myofasceitis syndrome (aluminum hydroxide), Gulf War syndrome (squalene), and post-vaccination phenomena (aluminum hydroxide). Other substances such as iodine, mercury, mineral oil, and titanium can also be associated with this syndrome2,3.
Silicone was first introduced to medicine, in 1947, in the use of dressings and, since then, it has been used in several materials and prostheses. Initially, it was considered an inert, stable substance, with a consistency that mimicked human tissue and resistant to degradation4,5.
After the beginning of the use of silicone in medicine, some studies pointed out that injectable silicone triggered severe local reactions and that they also appeared in places far from the implanted region, suggesting that the material was not immunologically inert as was believed6,7.
Evidence shows the association between breast implant placement and implant-related diseases since the 1960s, right after using silicone breast implants in reconstructions and aesthetic surgeries8.
The most common symptoms of ASIA Syndrome are arthritis, myalgia, fatigue, and neurological manifestations. Table 1 shows the possible symptoms9. These symptoms usually appear after a few years of the inclusion of the breast prosthesis2.
| Characteristics | Median or percentage |
|---|---|
| Age (years) | 43 (32-58) |
| BMI | 26 (22-29) |
| Symptom onset after breast prosthesis inclusion (years) | 8 (5-23) |
| Diabetes | 13% |
| Thyroidopathy | 30% |
| Prior allergy | 60% |
| Personal history of autoimmune disease | 30% |
| Family history of autoimmune disease | 20% |
To reach the diagnosis, there must be at least two major criteria or one major criterion and two minor symptoms of Table 2. The diagnosis is essentially clinical, through physical examination and medical history. There are no specific laboratory markers for the diagnosis of ASIA syndrome1.
| Symptoms (n) | Percentage of patients with symptom improvement (n) | |||
|---|---|---|---|---|
| 1 month | 3 months | 6 months | 12 months | |
| Myalgia (12) | 58% (7) | 67% (8) | 83% (10) | 92% (11) |
| Chronic fatigue (12) | 50% (6) | 67% (8) | 83% (10) | 83% (10) |
| Arthralgia or arthritis (11) | 36% (4) | 54% (6) | 73% (8) | 82% (9) |
| Dry skin and hair (7) | 43% (3) | 57% (4) | 86% (6) | 86% (6) |
| Headache (6) | 33% (2) | 50% (3) | 67% (4) | 67% (4) |
| Cognitive disorders (5) | 20% (1) | 60% (3) | 60% (3) | 100% (5) |
| Neurological manifestations (3) | 0% (0) | 33% (1) | 67% (2) | 67% (2) |
| Fever (2) | 0% (0) | 50% (1) | 100% (2) | 100% (2) |
| Depression (1) | 0% (0) | 0% (0) | 100% (1) | 0% (0) |
| Itching (1) | 0% (0) | 100% (1) | 100% (1) | 100% (1) |
Patients at the most significant risk of developing ASIA syndrome are those with a family history or history of autoimmune diseases, a history of allergy, atopic diseases, and vitamin D deficiency10.
The disease mechanism is related to inflammatory substances triggered by a foreign body that causes symptoms related to the disease11.
The treatment can be surgical only with the en bloc explant of the silicone breast prosthesis or be associated with medication such as corticosteroids, immunosuppressants, and biological medications; rarely, treatment will be drug-only12.
Quality of life assessment questionnaires have much helped to analyze and quantify the benefits of medical treatments. In breast surgeries, the Breast-Q® questionnaire has been used and even validated for use in the Portuguese language. Breast-Q® is an instrument for assessing body image and quality of life in patients undergoing breast surgery13-15.
Few studies in the medical literature assess the evolution of symptoms and quality of life in patients with ASIA syndrome treated exclusively with silicone breast implant explants.
OBJECTIVE
Describe the evolution of symptoms and quality of life of patients with ASIA syndrome who underwent silicone breast prosthesis explant.
METHODS
Fifteen sequential patients were operated on-demand from the office from January 2017 to December 2018. The Brazil Research Ethics Committee protocol number 33337520.0.0000.8054 was generated by Plataforma Brasil.
All patients were operated on by the author in São Paulo hospitals and had clinically confirmed ASIA syndrome. En bloc breast prosthesis explants and reconstruction with a mastopexy with an inverted T incision were performed in all patients.
The patients were followed up after one month, three months, six months, and one year postoperatively. In all returns, photographic records were made, and the evolution of symptoms related to ASIA syndrome was observed. The quality-of-life questionnaire used was the version translated into Portuguese of the Breast-Q Version 2.0® breast reduction module and applied only on the return of 1 year after surgery.
The Breast-Q® breast reduction module assesses the quality of life and patient satisfaction in 10 questionnaires. The questionnaires are on psychosocial well-being, sexual well-being, physical well-being, satisfaction with breasts, satisfaction with nipples, satisfaction with results, satisfaction with given information, satisfaction with the surgeon, satisfaction with the medical team, and satisfaction with the office staff.
As the Breast-Q® application manual guides it, it is unnecessary to apply all questionnaires; they can be used individually. Each questionnaire has a score from 0 to 100, and there is no sum of scores from all questionnaires.
In this study, questionnaires related to breast satisfaction, psychosocial well-being, sexual well-being, and physical well-being were applied.
The Breast-Q® questionnaires were authored by doctors Klassen, Pusic, and Cano and were developed under license from the Memorial Sloan Kettering Cancer Center, New York, USA13.
RESULTS
The demographic data of the studied sample are shown in Table 1.
The evolution of symptoms over the postoperative returns is shown in Table 2.
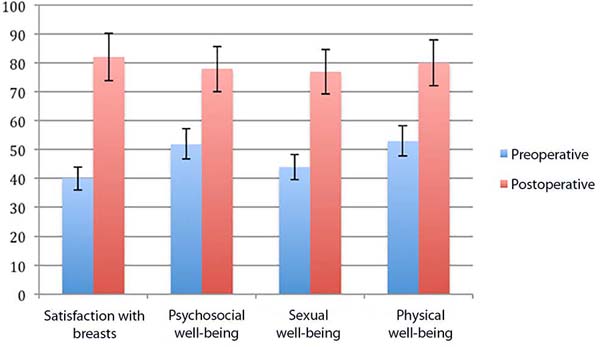
Figure 1 illustrates the scores of the Breast-Q® questionnaires before and after surgery.
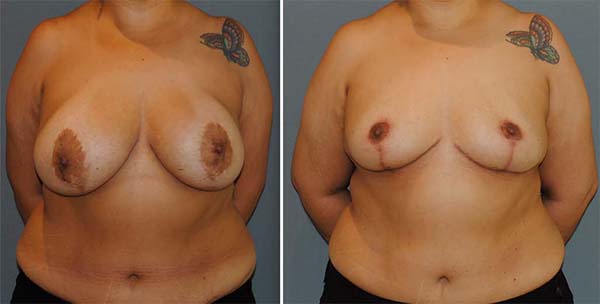
Figure 2 shows the explant of a patient who maintained the same breast prosthesis for nine years. In this patient, a fibroadenoma was also removed from the left breast. Figure 3 refers to the same patient with pictures of pre and postoperative six months.
DISCUSSION
It is necessary to clarify some terms used. Total capsulectomy involves the complete removal of the capsule; however, this term does not necessarily imply that the capsule and prosthesis will be removed en bloc. In total capsulectomy, the surgeon can open the capsule and remove the prosthesis, to later remove the entire capsule.
On the other hand, the en bloc explant involves removing the prosthesis and the capsule in a single piece, without breaking the capsule. It is the surgery recommended in the treatment of diseases associated with silicone implants.
The most significant criticism regarding the ASIA syndrome refers to the diagnostic criteria, which are not very specific and, on the other hand, are very comprehensive. Thus, the diagnostic criteria can include many patients with other autoimmune diseases and a large population with these symptoms without any autoimmune disease16.
Studies show that breast prosthesis explant is not a guarantee of improvement in ASIA syndrome symptoms. There are patients whose symptoms definitely improve after the explant; others whose symptoms temporarily improve, and those in which the symptoms do not improve11,12.
The most common symptoms, such as myalgia, arthralgia, chronic fatigue, dry skin, and hair, improved in more than 80% of patients operated on after 12 months of follow-up in the sample studied. There was an improvement in 100% of patients’ cognitive impairment, fever, and pruritus on returning after 12 months of surgery.
The percentage of patients who improved showed a tendency to increase until six months after surgery then remained stable until 12 months.
In the patient who had depression, there was an improvement at six months of follow-up and then a relapse at 12 months. It can be inferred that the surgery had some transient placebo effect on the patient or that the breast prosthesis was not related to depression in this case.
In the graph of the score of the Breast-Q®questionnaires, it can be seen a significant increase in the postoperative period compared to the preoperative one. Although no statistical analysis has been carried out, the improvement is evident to the point that there is no overlap of the score between the pre and the postoperative period, even considering the standard deviation of the graphs. There was an improvement in the quality of life and satisfaction with the breasts after the surgical procedure. The score was very close among the four postoperative questionnaires. The lowest scores in the preoperative period were in the satisfaction questionnaires with breasts and sexual well-being.
The sample studied demographic data showed patients with a history of allergies, a personal and family history of autoimmune diseases, and the onset of symptoms on average eight years after the inclusion of the breast prosthesis. These characteristics corroborate medical literature10.
The mechanism by which symptoms may improve after the explant is reduced inflammatory response as there is no longer silicone stimulation.
However, many patients may still experience symptoms even after the explant. This occurs due to silicone presence in the lymph nodes and other body organs due to the migration of cells containing silicone. In these cases, the silicone is still present, and the inflammatory stimulus continues even after the explant.
The medical literature points out that the longer the period that patients have symptoms related to ASIA syndrome, the lower the chance that surgery alone will improve symptoms. In these cases, drug treatment with immunosuppressants may be necessary for conjunction with surgery11,12.
There are some aspects of this study that must be considered. The first is the sample size, which may not be representative of the population studied. The second question is that statistical tests of correlation between the explant and the improvement of symptoms and quality of life were not performed. Variables can be associated but have no causal relationship.
It is necessary to clarify to the patient that only the breast prosthesis explant may not improve the syndrome symptoms. Besides, there are implications for removing the prosthesis, such as loss of the breast’s aesthetic result, the possibility of additional scarring, and complications related to any surgery.
CONCLUSION
Breast prosthesis explant in patients with ASIA syndrome appears to be associated with improved quality of life and decreased symptoms related to the syndrome.
Further studies with a larger sample and statistical analysis are needed to investigate the causal correlation between breast prosthesis explants and improved quality of life, and decreased symptoms.
REFERENCES
1. Shoenfeld Y, Agmon-Levin N. ASIA - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimm. 2011 Fev;36(1):4-8.
2. Jara LJ, García-Collinot G, Medina G, Cruz-Dominguez MDP, Vera-Lastra O, Carranza-Muleiro RA, et al. Severe manifestations of autoimmune syndrome induced by adjuvants (Shoenfeld's syndrome). Immunol Res. 2017 Fev;65(1):8-16.
3. Perricone C, Alessandri C, Valesini G. 'ASIA' - autoimmune/inflammatory syndrome induced by adjuvants: even and odd. Reumatismo. 2011;63(2):63-6.
4. Brown JB, Fryer MP, Randall P, Lu M. Silicones in plastic surgery; laboratory and clinical investigations, a preliminary report. Plast Reconstr Surg (1946). 1953 Nov;12(5):374-6.
5. Brown J, Fryer M, Ohlwiler DA. Study and use of synthetic materials, such as silicones and teflon, as subcutaneous prostheses. Plast Reconstr Surg. 1960;26:264-79.
6. Winer LH, Sternberg TH, Lehman R, Ashley Fl. Tissue reactions to injected silicone liquids: a report of three cases. Arch Dermatol. 1964 Dez;90:588-93.
7. Bridges AJ, Vasey FB. Silicone breast implants. History, safety, and potential complications. Arch Intern Med. 1993 Dez;153(23):2638-44.
8. Miyoshi KMT, Kobayashi Y. Hypergammaglobulinemia by prolonged adjuvanticity in men. Disorders developed after augmentation mammaplasty. Jpn Med J. 1964;2122:9-14.
9. Colaris MJL, Boer M, Van Der Hulst RR, Tervaert JWC. Two hundreds cases of ASIA syndrome following silicone implants: a comparative study of 30 years and a review of current literature. Immunol Res. 2017 Fev;65(1):120-8.
10. Goren I, Segal G, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvant (ASIA) evolution after silicone implants. Who is at risk?. Clin Rheumatol. 2015 Out;34(10):1661-6.
11. Shoenfeld Y. Video Q&A: what is ASIA? An interview with Yehuda Shoenfeld. BMC Med. 2013;11:118.
12. Boer M, Colaris M, Van Der Hulst RRWJ, Tervaert JWC. Is explantation of silicone breast implants useful in patients with complaints?. Immunol Res. 2017 Fev;65(1):25-36.
13. Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009 Aug;124(2):345-53.
14. Sbalchiero JC, Cordanto-Nopoulos FR, Silva CHD, Caiado Neto BR, Derchain S. Breast Q questionnaire, translation process to portuguese language and their application on breast cancer patients. Rev Bras Cir Plást. 2013;28(4):549-52.
15. Côrrea MPD, Costa AMD, Côrrea LD, Dornelas MT, Venturelli Júnior EP, Chaoubah A. Assessment of the quality of life in patients with breast hypertrophy before and after reduction mammoplasty. Rev Bras Cir Plást. 2019;34(2):204-9.
16. Tervaert JWC. Autoinflammatory/autoimmunity syndrome induced by adjuvants (ASIA; Shoenfeld's syndrome): a new flame. Autoimmun Rev. 2018 Dez;17(12):1259-64.
1. Private Clinic, Plastic Surgery, São Paulo, SP, Brazil.
Corresponding author: Ricardo Eustachio de Miranda, Rua Bandeira Paulista, 530, Itaim Bibi, São Paulo, SP, Brazil. CEP: 04532-001. E-mail: ricardomiranda@hotmail.com
Article received: May 07, 2020.
Article accepted: July 19, 2020.
Conflicts of interest: none






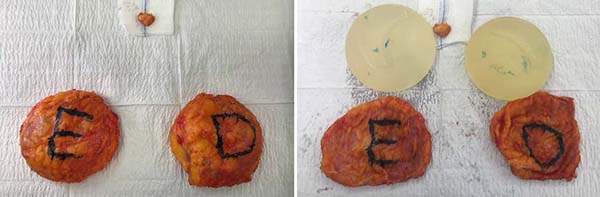

 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter