

Original Article - Year 2020 - Volume 35 -
Distant flap transfer with the vascular pedicle section in second stage
Transferência de retalho à distância com secção do pedículo vascular em segundo tempo
ABSTRACT
Introduction: Occasionally, there is no possibility of covering a
wound with local grafts or flaps. This study aimed to evaluate
the capacity of the distant flap transfer with the vascular
pedicle section in second stage to cover the region that suffered
substance loss.
Methods: Five patients with substance loss, with
no reconstruction option using grafts or local flaps, had their
wounds covered by distant flap transfer with the vascular pedicle
section in second stage.
Results: The transferred flaps were
useful in covering the wounds.
Conclusion: distant flap transfer
with the vascular pedicle section in second stage is a simple and
effective procedure. Every plastic surgeon must master that.
Keywords: Multiple trauma; Wounds and injuries; Plastic surgery; Wound closure techniques; Autologous transplantation.
RESUMO
Introdução: Ocasionalmente não há possibilidade de se cobrir uma ferida com enxertos ou retalhos locais. O objetivo deste trabalho foi avaliar a resolutividade da transferência de retalho à distância com secção do pedículo vascular em segundo tempo para cobertura de região que sofreu perda de substância.
Métodos: Cinco pacientes com perda de substância, sem opção de reconstrução com enxertos ou retalhos locais, tiveram suas feridas cobertas por retalhos transferidos à distância, com secção dos pedículos vasculares em segundo tempo.
Resultados: Os retalhos transferidos foram eficazes na cobertura das feridas.
Conclusão: A transferência de retalho à distância com secção do pedículo vascular em segundo tempo é procedimento simples e eficaz, que deve ser dominado por todo cirurgião plástico.
Palavras-chave: Traumatismo múltiplo; Ferimentos e lesões; Cirurgia plástica; Técnicas de fechamento de ferimentos; Transplante autólogo
INTRODUCTION
In plastic surgery, grafts and flaps are used to move tissues and repair defects. Occasionally, the condition of a wound and its surroundings do not favor its coverage with a graft or local flap. Distant flaps transfer can be good options in these cases. In the 15th century, the Branca family members made a flap on the arm and transferred it directly to reconstruct a nasal defect. Members of the Vianeo family have optimized this method by adopting a time interval between making and definitive transfer. Tagliacozi expanded and disseminated this concept in the 16th century1,2. In 1854, Hamilton made the first cross leg flap to treat a patient with a chronic ulcer. In 1862, Wood made the first axial distant flap in the inguinal region. In 1950, Gudin and Pangman described the cross finger flap, but Cronin had already made it in 1945 during World War II3.
The importance of distant flap transfer with the vascular pedicle section in second stage has decreased due to better knowledge of the vascular pattern and angiosomes. The development of other techniques brought alternatives, such as axial, fasciocutaneous, musculocutaneous flaps, flaps based on perforating vessels, and free flaps. However, distant flap transfer with the vascular pedicle section in second stage remains valid for many flaps frequently used for reconstruction of the head and neck, forearm, hand, leg, and foot4.
OBJECTIVE
This study aimed to evaluate the capacity of the distant flap transfer with section of the vascular pedicle in second stage to cover the region that suffered loss of substance.
METHODS
Based on the author’s series of cases, this observational study describes technical aspects of the indication, execution, and monitoring of the distant flap transfer with the vascular pedicle section in the second stage, used for aesthetic and functional reconstruction. No interventions were performed other than those necessary and enshrined in the literature for care treatment. There are no conflicts of interest or sources of funding.
Patients were treated in Belo Horizonte / MG, at Hospital João XXIII, and in Betim / MG, at Hospital Regional de Betim, between 2007 and 2019.
Adult patients were selected, regardless of gender, who suffered tissue loss for reconstruction by the distant flap transfer with section of the vascular pedicle in second stage.
The exclusion criteria for distance flap reconstruction were:
1. Infection carriers;
2. Possibility of primary synthesis of the lesion;
3. Possibility of adequate reconstruction with skin grafting;
4. Possibility of adequate reconstruction with local flap;
5. Patient’s refusal of the proposed treatment.
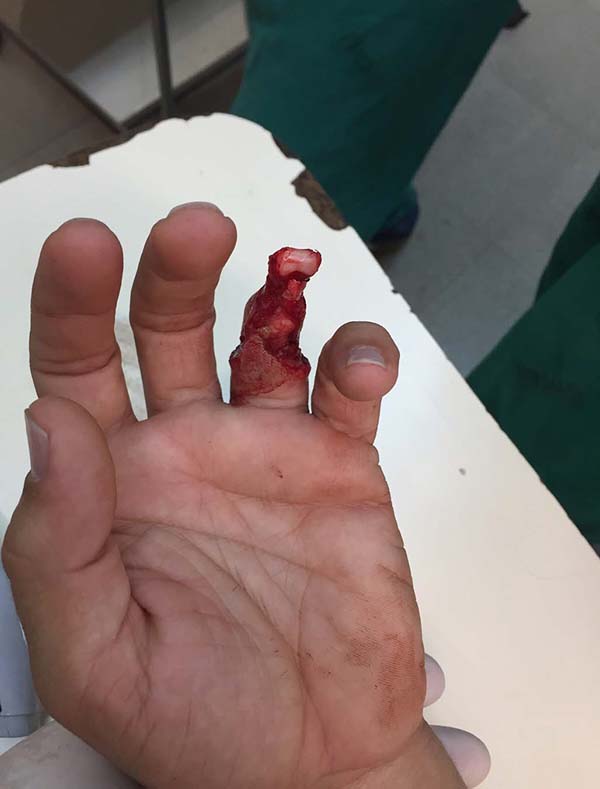
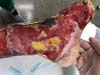
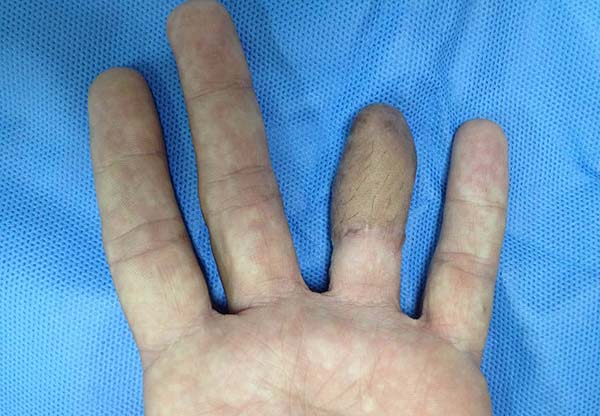
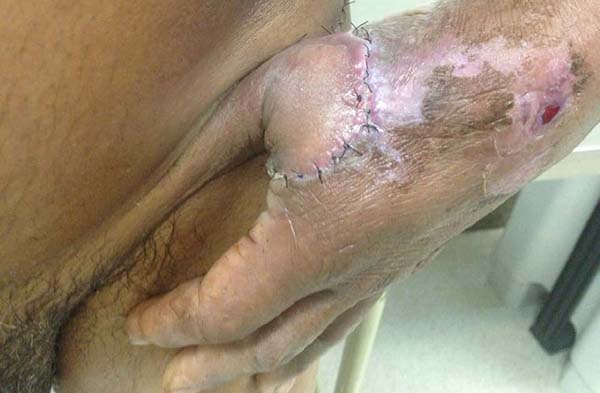
Patient 1: victim of amputation of the distal phalanx and degloving of the fourth finger of the left hand by an wedding ring attached to a goal post during a soccer match, preserving the movement of the superficial flexor tendon; there is cartilaginous exposure (Figure 1).
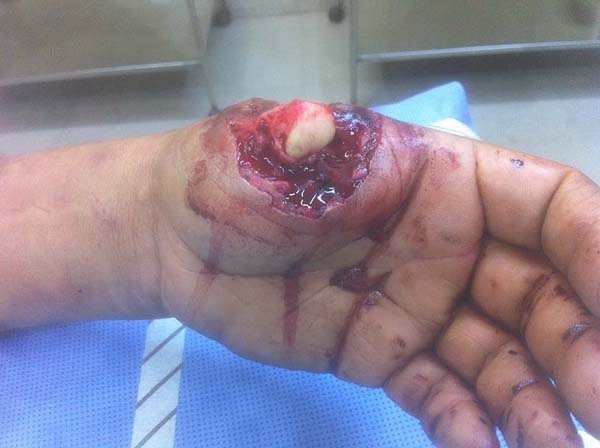
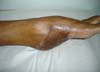
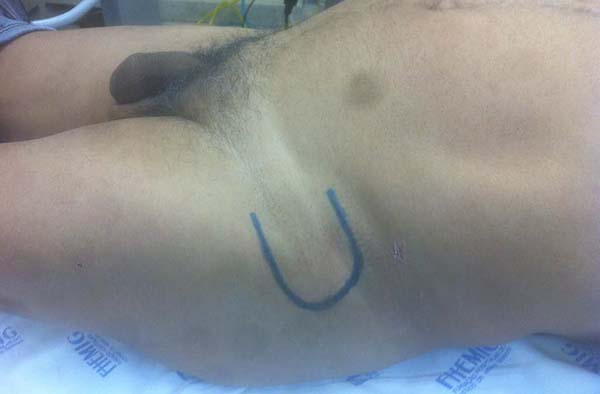
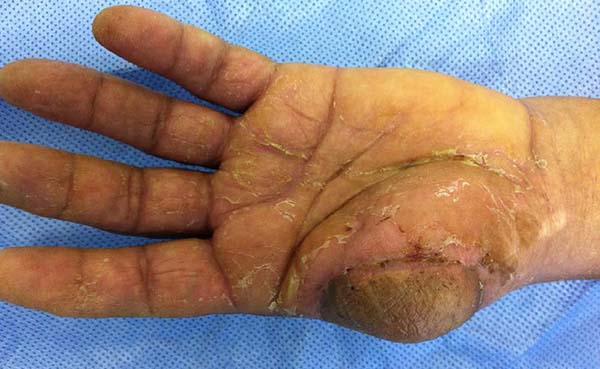
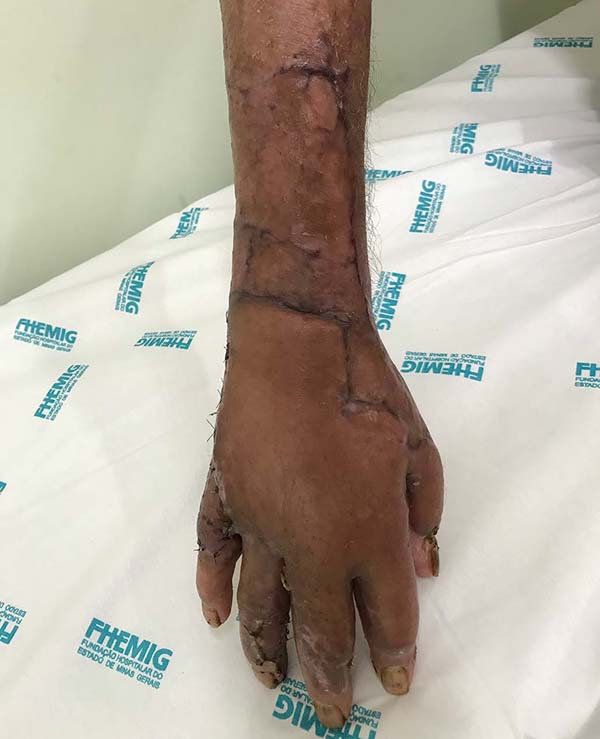
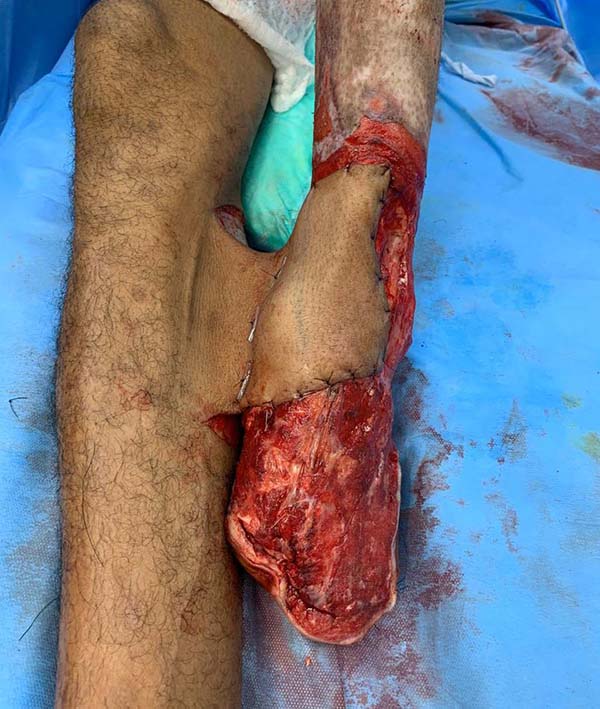
Patient 2: victim of trauma by drill, with amputation of the left thumb due to pullout along with the protective glove, and loss of peripheral substance. The rudimentary pincer movement through the first metacarpal was maintained; there was bone exposure without periosteum (Figure 2).
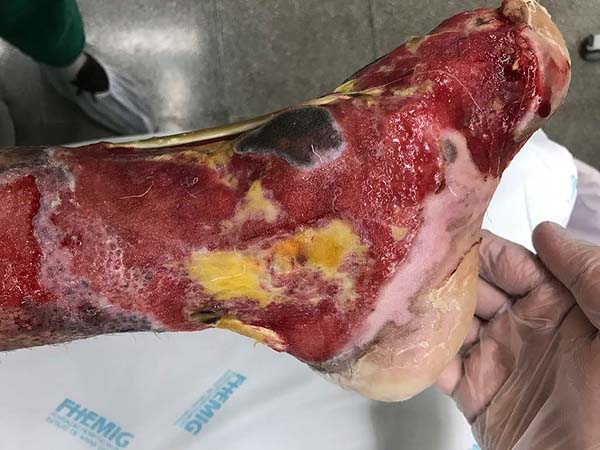
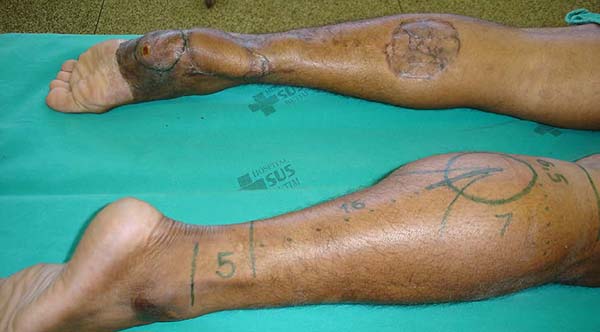
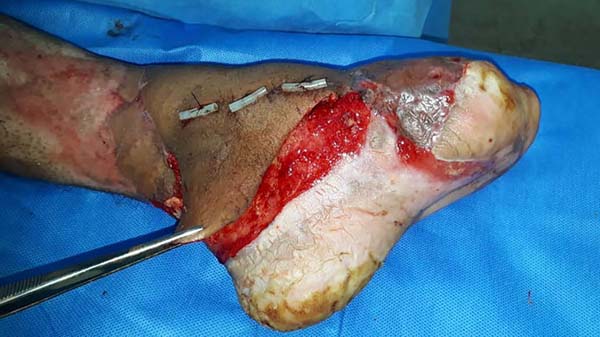
Patient 3: Patient 3: victim of being hit by a car years ago, with loss of the left calcaneus and loss of partial plantar substance of the left foot. At that time, he underwent partial skin grafting and evolved with fixed plantar flexion of the foot (Figure 3).
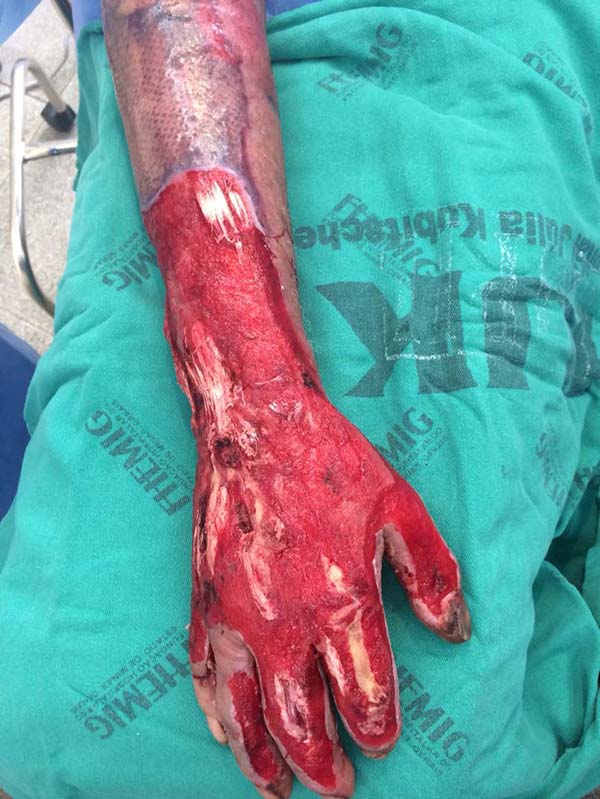
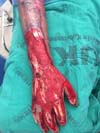
Patient 4: victim of thermal burn. There is bone exposure without periosteum and tendon without peritenon (Figure 4).
Patient 5: victim of a thermal burn, with significant loss of substance. Several tendons are exposed without peritenon (Figure 5).
An attempt was made to choose a tissue donor region with the possibility of wide movement to reach the recipient region without tension, cover it entirely, and minimize discomfort. It was necessary to immobilize the treated segment during 21 days to establish the receptor region’s vascular connection before the pedicle section.
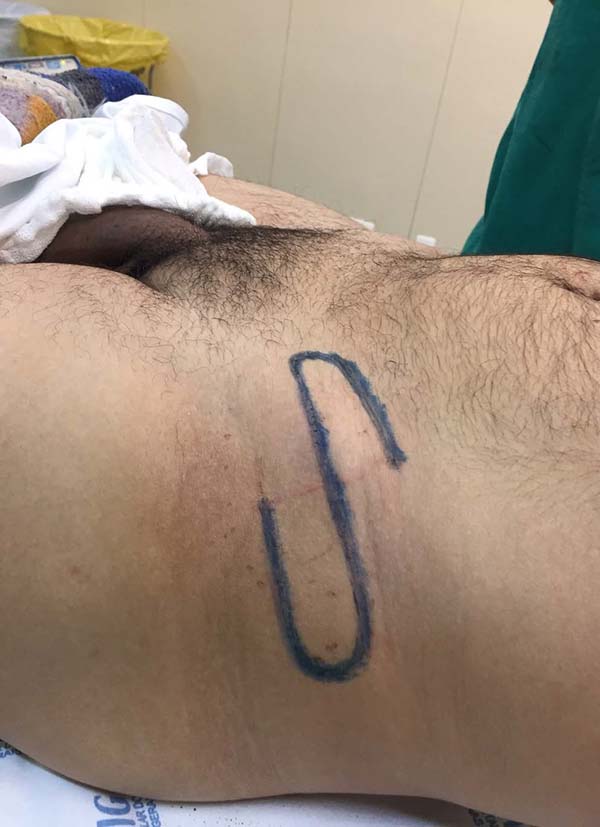
The area of the region to be covered was measured to estimate the necessary amount of tissue to be recruited to allow the donor region’s primary synthesis or skin grafting. In cases of need for a very long vascular pedicle, its length was estimated using a cord made of gauze, as a necessary bridge to carry the flap and unite the donor and recipient regions without tension, and in the shape of a soft arch, without sudden change of direction. In the donor region, the flap area to be transferred and the contiguous tissue segment that would exercise the vascular pedicle’s function was demarcated, according to the cord’s length made with gauze, taking into account the patient’s comfort during the transfer period. We tried to limit the pedicle’s width to about half the total length dimension in randomly based flaps. In more extended flaps, axial vessels were included.
Considering the location to be treated and the flap’s donor region, spinal anesthesia or general anesthesia was performed, as well as antimicrobial prophylaxis, the perimeter was incised, and the flap to be transferred was elevated. The flap was approached to the region to be covered without tension or acute angulation of its pedicle. The flap was fixed in position by simple, cardinal, absorbable points on the dermis and simple nylon points on the skin. The primary synthesis of the flap donor region was carried out similarly. If primary synthesis was impossible, skin grafting was performed on the tissue donor region.
In general, the vascular pedicle’s bloody face was protected by wrapping it with sterile gauze soaked in oil. If necessary, the segment containing the recipient region, the donor region, and its vascular pedicle was immobilized with orthopedic cotton and plaster bandages. This measure sought to minimize the flap movement in the process of connecting blood vessels to the recipient region. The region under treatment was cleaned, and the dressings and immobilization were changed weekly until 21 days were completed. During this period, patients required to remain in bed received prophylaxis against venous thromboembolism with heparin.
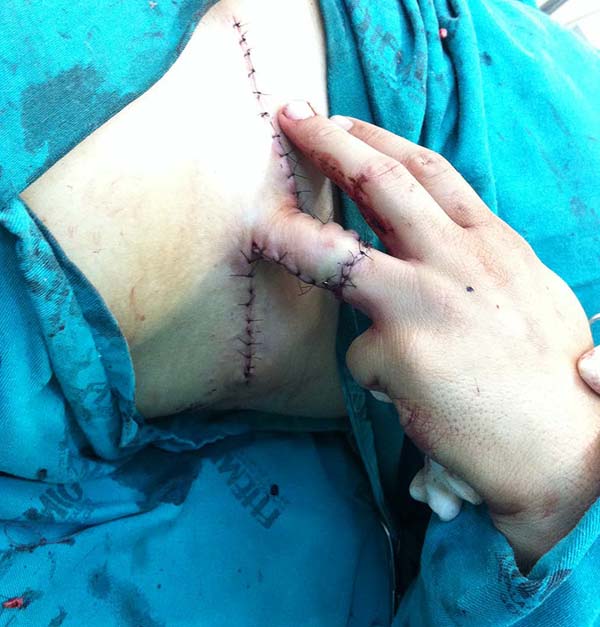
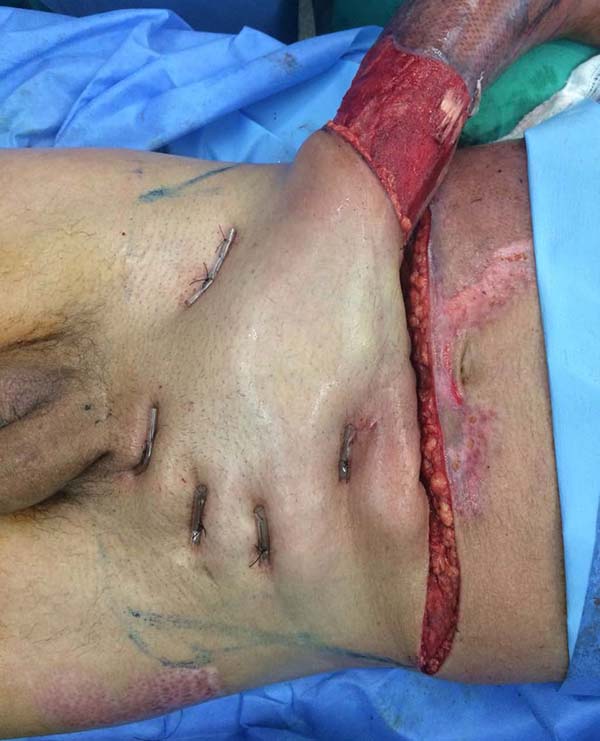
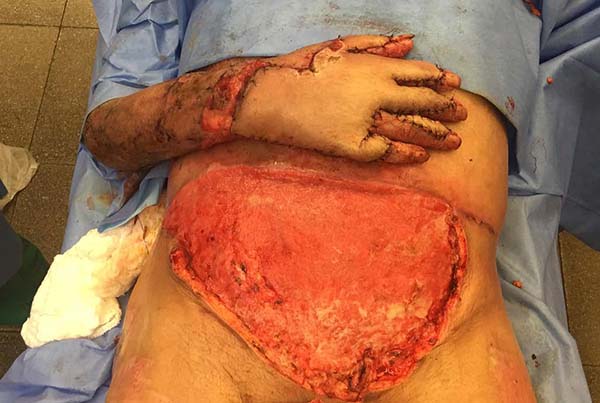
After three weeks, the vascular pedicle was sectioned on the edge of the transferred flap, and fixation of the flap in the recipient region was completed with nylon thread. The proximal segment, which contained the sectioned pedicle, was discarded, either re-fixed at its origin with nylon thread or used to cover another noble structure’s exposure, contiguous to the transferred flap. At that point, the sutures from the previous procedure were removed. The dry dressing was applied. The sutures of this new procedure were removed in three weeks. Due to joint immobilization for 21 days, patients were referred to physiotherapy.
RESULTS
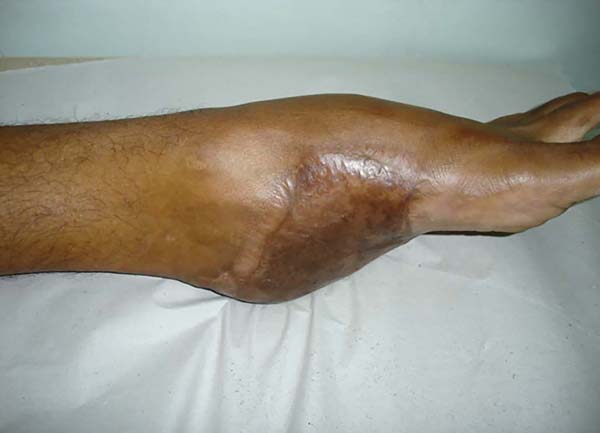
Patient 1: the finger wound was covered with a flap from the inguinal region (Figures 6 to 8). Patient 2: the hand-wound was covered with a flap from the inguinal region (Figures 9 to 11). Patient 3: In the first approach, he underwent an incision in the grafted region’s proximal margin, the release of adhesions, and elongation of the calcaneus tendon by sliding, to enable dorsiflexion of the foot. The exposed calcaneous tendon, without a peritenon, was covered by a reverse flow sural fasciocutaneous flap on the same side. In the second approach, the patient underwent surgical debridement of the ulcer caused by the body’s support over the fragile old partial skin graft from the calcaneus, which started to touch the soil after the first approach, and which was partially removed. He was then submitted to this region’s coverage by a contralateral reverse flow sural fasciocutaneous flap (Figures 12 to 14).
Patient 4: To cover the backs of the hand and forearm, and abdominal skin flap with a lower base was made since the region that would be the flap’s upper base was grafted due to the burn. The limb to be covered was buried under the flap, and the fingers were individualized by plastic segments of the urethral tube, held in position to shape the interdigital folds, fixed by points that joined the tube to the abdominal aponeurosis, transfixing the skin (Figures 15 to 17).
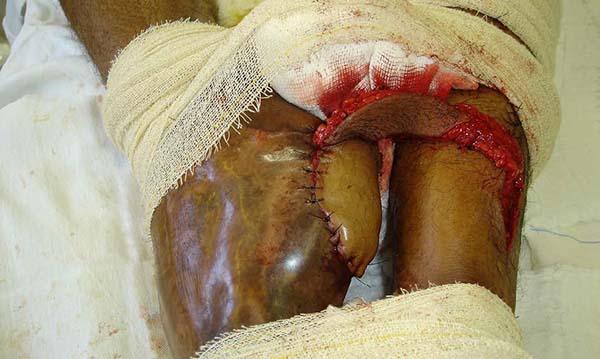
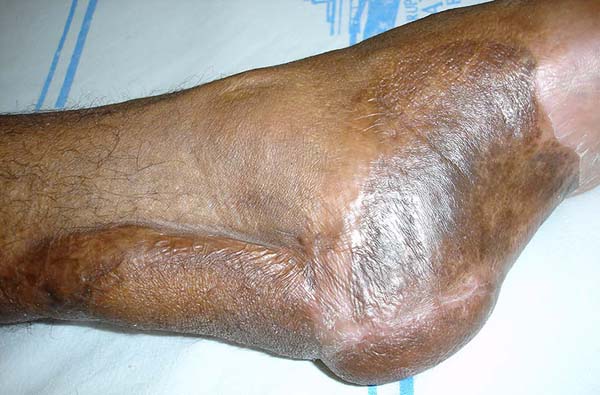
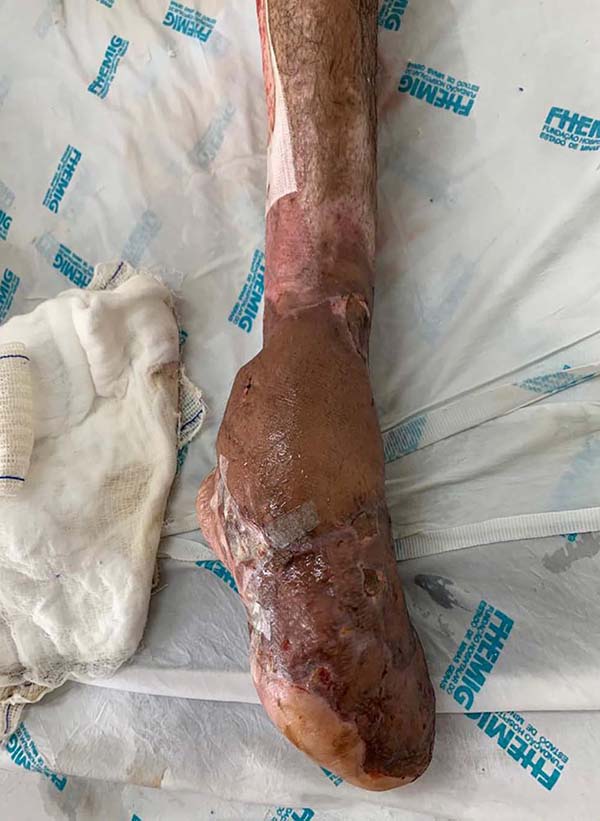
Patient 5: the tendons in the anterior ankle and foot were covered with a flap from the contralateral leg. Urethral tube segments were used in the transition region between the vascular pedicle and the flap to be transferred, sutured to the deep plane to reinforce the flap surface’s integral contact with the recipient bed. After flap transfer and section of the vascular pedicle, the excess tissue, based on the transferred flap, was used to cover the calcaneus tendon (Figures 18 to 20).
The transferred flaps were useful in covering the wounds. There were no cases of necrosis or infection in the donor or recipient regions).
DISCUSSION
The distant flap transfer with the vascular pedicle section in second stage has been significantly used for years. Despite the need for a second surgical procedure and the patient’s discomfort, the treated region remains immobilized for weeks; it allows more complex tissues than grafts to be transferred between non-contiguous locations. The selection of adult patients was justified by understanding the temporary discomfort and cooperation during this period. To reduce discomfort, one should seek more comfortable positions without wide angles, without friction between limbs, which may cause skin maceration, and with a loose pedicle of the flap, which prevents the patient’s small movements from causing damage to the fragile connections in formation between donor and recipient blood vessels. To adopt this strategy concerning patient 3, we opted for an axial flap with contralateral reverse flow instead of anterograde flow to avoid significant flexion of one of the legs and contact between them.
The transferred flap does not have immediate nutrition from the receiving bed, allowing it to cover noble structures without blood supply on its surface, such as bone without periosteum, cartilage without perichondrium, tendon without peritenon or nerve without perineurum. These were the reasons that made reconstruction impossible just by skin grafting. In all cases, arterial bleeding was noted at the time of incision of the vascular pedicle after 21 days, without the need for any previous viability test.
Often the tissues close to injuries caused by trauma are also damaged, making it challenging to plan reconstructions with local flaps. Also frequent are orthopedic external fixators, which limit the selection of the most appropriate local flap. It should be avoided to include hair in the donor region’s demarcation if they do not exist in the recipient region, which could have been observed in patient 1.
A different aspect is the vascular safety of a flap. For years, it has been stimulated the idea that vascular safety could be increased by reducing the flap’s blood supply in stages. In 1921, Blair defined this process as flap delay5-7. It enhances the vascular viability of the flap when subjected to a period of ischemia before its elevation. Its objective is to increase its survival chances after the elevation, regardless of whether it will be moved to cover a contiguous region or a distant one. There are several techniques proposed to cause ischemia, such as ligation of blood vessels, incisions around the perimeter of the proposed flap, or its isolation from the deep plane, but not its elevation, as in this case, the flap would have a reduction in its blood supply instantly and would not benefit from the enhancement of vascular viability8,9.
Even with the emergence of new techniques, distant flap transfer remains a safe and easy alternative. Versatile, it must be part of the arsenal of every plastic surgeon, who will be able to expand his resolving capacity to treat complex defects, as he often does not have access to resources, such as negative pressure therapy, dermal matrix, or training and structure to perform microsurgery, mainly in public hospitals.
CONCLUSION
The distant flap transfer with the vascular pedicle section in second stage effectively covers regions that have suffered substance loss without good local options.
ACKNOWLEDGMENT
I would like to thank Professor Doctor Armando Chiari Júnior, full member of the Sociedade Brasileira de Cirurgia Plástica, for the precision in the concept’s definition in plastic surgery.
REFERENCES
1. Greco M, Ciriaco AG, Vonella M, Vitagliano T. The primacy of the Vianeo Family in the invention of nasal reconstruction technique. Ann Plast Surg. 2010 Jun;64(6):702-5.
2. Whitaker IS, Karoo RO, Spyrou G, Fenton OM. The birth of plastic surgery: the story of nasal reconstruction from the Edwin Smith Papyrus to the twenty-first century. Plast Reconstr Surg. 2007 Jul;120(1):327-36.
3. Fang F, Chung KC. An evolutionary perspective to the history of flap reconstruction in the upper extremity. Hand Clin. 2014 Mai;30(2):109-22.
4. Ghali S, Butler PE, Teper OM, Gurtner GC. Vascular delay revisited. Plast Reconstr Surg. 2007;119(6):1735-44.
5. Kubulus D, Roesken F, Amon N, Rücker M, Bauer M, Bauer I, et al. Mechanism of the delay phenomenon; tissue protection is mediated by heme oxygenase-I. Am J Physiol Heart Circul Physiol. 2004 Nov;287(5):H2332-40.
6. Blair VP. The delayed transfer of long pedicle flaps in plastic surgery (face). Surg Gynecol Obstetr. 1921;33:261-72.
7. Milton SH. The effects of "delay" on the survival of experimental pedicled skin flaps. Br J Plast Surg. 1969;22(3):244-52.
8. Lineaweaver WC, Lei MP, Mustain W, Oswald TM, Cui D, Zhang F. Vascular endothelium growth factor, surgical delay, and skin flap survival. Ann Surg. 2004 Jun;239(6):866-75.
9. Ribeiro L, Pessoa MCM, Rocha RB. Autonomização da cicatriz umbilical: técnica segura para abdominoplastias secundárias. Rev Bras Cir Plást. 2011;26(3):488-95.
1. Hospital João XXIII, Plastic Surgery, Belo Horizonte, MG, Brazil.
RPS Analysis and/or data interpretation, Conception and design study, Conceptualization, Data Curation, Final manuscript approval, Investigation, Methodology, Project Administration, Realization of operations and/or trials, Validation, Writing - Original Draft Preparation, Writing - Review & Editing
Corresponding author: Rodrigo Pimenta Sizenando, Avenida das Constelações, 725, Prédio 3, Apto. 302, Nova Lima, MG, Brazil. Zip Code: 34008-050. E-mail: rodrigosizenando@hotmail.com
Article received: July 12, 2019.
Article accepted: July 19, 2020.
Conflicts of interest: none







 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter