

Case Report - Year 2020 - Volume 35 -
Treatment of deep second-degree burns on the abdomen, thighs, and genitalia: use of tilapia skin as a xenograft
Tratamento de queimaduras de segundo grau profundo em abdômen, coxas e genitália: uso da pele de tilápia como um xenoenxerto
ABSTRACT
Tilapia skin has a non-infectious microbiota and a morphological structure similar to human skin. Phase II clinical studies, not yet published, have shown promising results in their use for the treatment of burns. In the protocols of these studies, patients with lesions in areas of skin folds, such as genitals and inguinal regions, were excluded, as it was thought that the biomaterial would not adhere properly, resulting in a lower degree of healing. Case report of a female patient, 18 years old, without comorbidities, with deep second-degree burns in the abdomen, inguinal region, part of the genitalia and upper half of both thighs, involving 13.5% of the total body surface area. Tilapia skin was applied to the lesions leading to a complete re-epithelialization with 16 days of treatment. No side effects were observed. Tilapia skin, therefore, brings the promise of an innovative product, easy to apply, and highly available, which can become the first animal skin nationally studied and registered by the Agência Nacional de Vigilância Sanitária, for use in the treatment of burns. This case report contributes to reduce the limitations concerning the anatomical areas appropriate for the application of tilapia skin, since, even with the need for skin replacement, good results were obtained with application to the genitalia and inguinal region.
Keywords: Burns; Tilapia; Biological dressings; Biocompatible materials; Cichlids.
RESUMO
A pele de tilápia possui microbiota não infecciosa e estrutura morfológica semelhante à pele humana. Estudos clínicos fase II, ainda não publicados, mostraram resultados promissores na sua utilização para tratamento de queimaduras. Nos protocolos destes estudos, pacientes com lesões em áreas de dobras de pele, como genitais e região inguinal, foram excluídos, pois achava-se que o biomaterial não aderiria apropriadamente, resultando em um grau de cicatrização inferior. Relato de caso de paciente do sexo feminino, 18 anos, sem comorbidades, com queimaduras de segundo grau profundo em abdômen, região inguinal, parte da genitália e metade superior de ambas as coxas, envolvendo 13,5% da área total da superfície corporal. A pele de tilápia foi aplicada nas lesões levando a uma reepitelização completa com 16 dias de tratamento. Não foram observados efeitos colaterais. A pele de tilápia traz, portanto, a promessa de um produto inovador, de fácil aplicação e alta disponibilidade, que pode se tornar a primeira pele animal nacionalmente estudada e registrada pela Agência Nacional de Vigilância Sanitária, para uso no tratamento de queimaduras. Este relato de caso contribui para reduzir as limitações em relação às áreas anatômicas apropriadas para a aplicação da pele de tilápia, uma vez que, mesmo com a necessidade de reposição de pele, foram obtidos bons resultados com aplicação na genitália e região inguinal.
Palavras-chave: Queimaduras; Tilápia; Curativos biológicos; Materiais biocompatíveis; Ciclídeos
INTRODUCTION
Burns are responsible for 180,000 deaths annually, which are mainly concentrated in low and middle-income countries, a group in which Brazil is included. Besides, non-fatal burns result in prolonged hospitalization, disfigurement, and disability, with subsequent stigma and rejection1.
Nile tilapia (Oreochromis niloticus) is the most cultivated fish in Brazil and the fourth most cultivated in the world2. In addition to the full availability and constituting a product that used to be discarded, tilapia skin demonstrated, in previous studies, a non-infectious microbiota3, a morphological structure similar to human skin, even with higher amounts of collagen type14.5, and excellent results when it was used as a xenograft to treat experimental burns in rats6.
Phase II clinical studies, not yet published, comparing tilapia skin with 1% silver sulfadiazine cream have shown promising results. In the protocols of these studies, patients with burns in areas such as the face, genitals, neck, armpits, antecubital fossa, and inguinal region were excluded. The presence of skin folds in these regions generated the hypothesis that the biomaterial would not adhere properly, resulting in a lower degree of healing.
OBJECTIVE
Report the case of a patient with deep second-degree burns involving genitalia and inguinal region, among other areas, in which treatment was performed using tilapia skin as a xenograft.
CASE REPORT
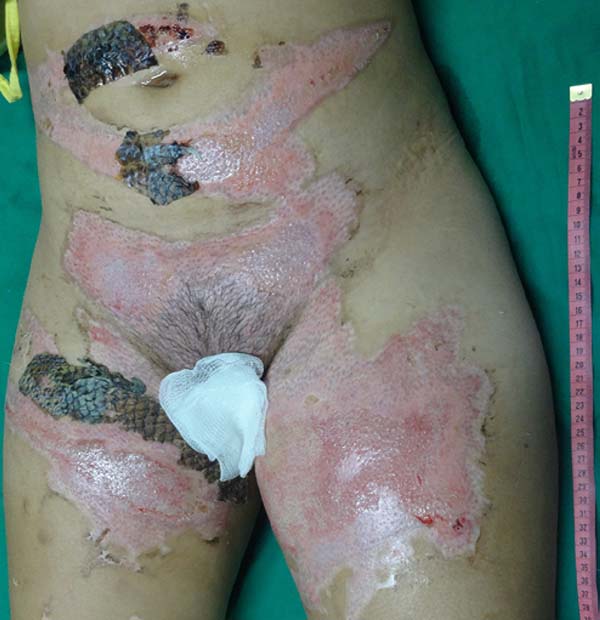
Female patient, 18 years old, without comorbidities, admitted to a burn unit after thermal injury by direct contact with flames. Using the Lund and Browder table, an involvement of 13.5% of the total body surface area (TBSA) was calculated, mostly by deep second-degree burns and, less significantly, by superficial second-degree burns (Figure 1). After hospitalization, the patient was resuscitated with intravenous fluids according to Parkland’s formula and remained hemodynamically stable. Approval from the Research Ethics Committee and written permission from the patient were obtained.
The method of processing, decontamination, and sterilization of tilapia skin for use in burns was registered at the Instituto Nacional da Propriedade Industrial (INPI) under number BR1020150214359 and is described in Lima Júnior et al., In 20176. Before use in the patient, the skin was washed in sterile 0.9% sodium chloride solution for 5 minutes, a process that is repeated three times in a row. The coverage of at least 1cm of healthy skin at the edges of the burned area and the overlapping of at least 1cm between the pieces of skin are necessary procedures to ensure that an eventual movement of patients in the first days of treatment does not lead to the exposure of any segment of the lesion.
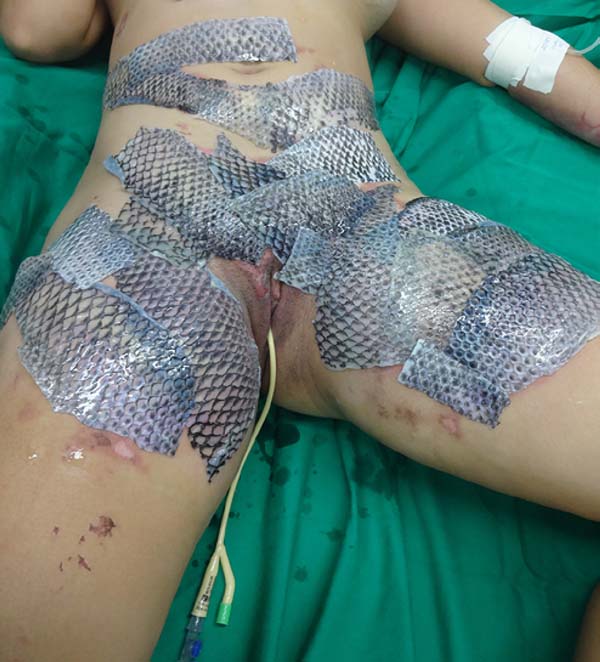
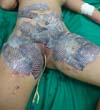
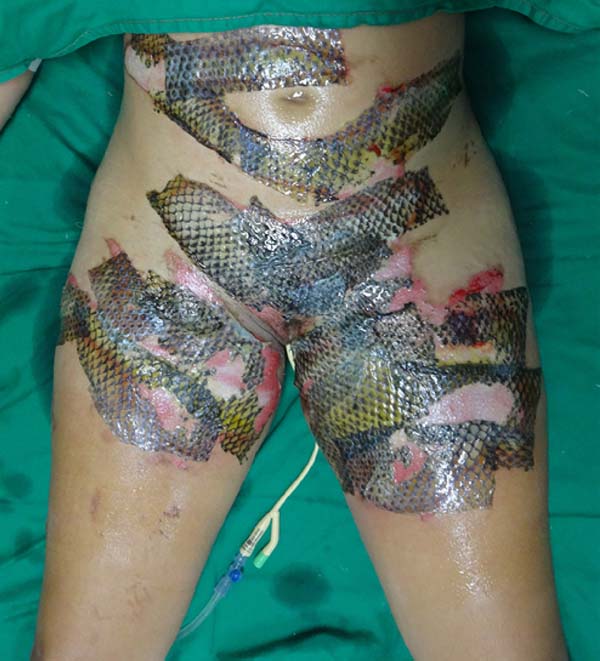
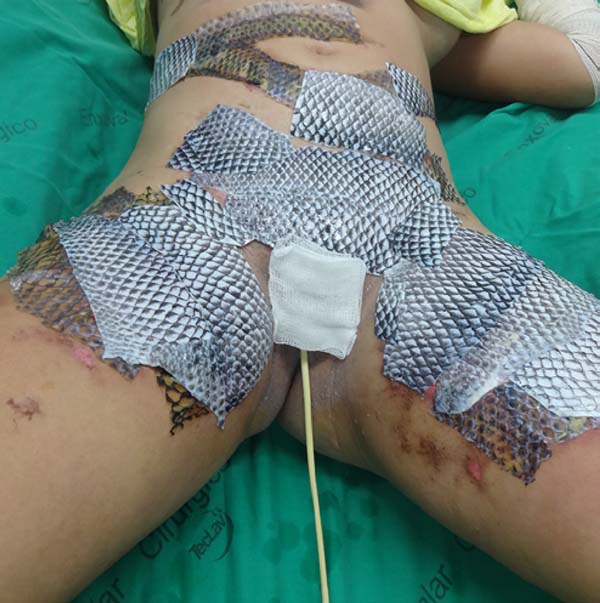
The patient underwent balneotherapy under anesthesia and analgesia with 100mg of ketamine, 2mg of midazolam, and 2000mg of dipyrone. After removing any necrotic tissue and cleaning the lesion with drinking water and 2% chlorhexidine gluconate, the tilapia skin was applied. In total, 11 tilapia skins were used, sometimes cut to fit the contour of the burned area (Figure 2). Finally, the region was firmly covered with gauze and bandage.
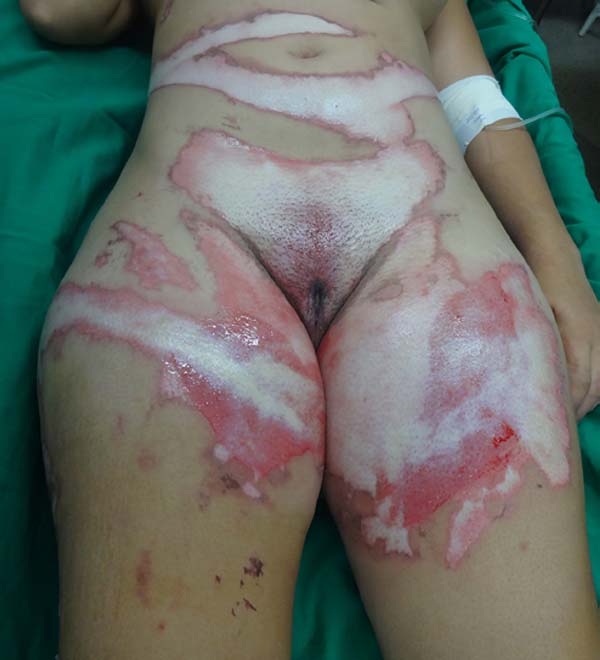
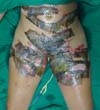
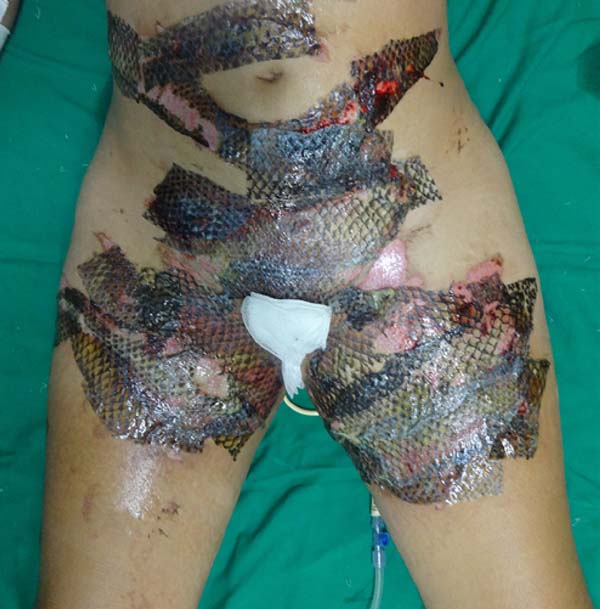
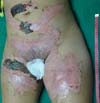
On the fourth day of treatment, the patient was submitted to a new anesthetic bath, in which the dressing was opened for the first time. It was observed good adhesion of the tilapia skin in part of the burned surface; however, in some regions, the skin did not adhere, having been removed together with the gauze or remaining in the burn bed, but with softened consistency and excess of underlying secretion (Figure 3). In these regions, the tilapia skin was removed (Figure 4), and, after cleaning, the biomaterial was replaced (Figure 5), covering tightly with gauze and bandage. The same sequence of procedures was performed on the seventh day of treatment, but with better adherence to the tilapia skin and less presence of secretion (Figure 6).
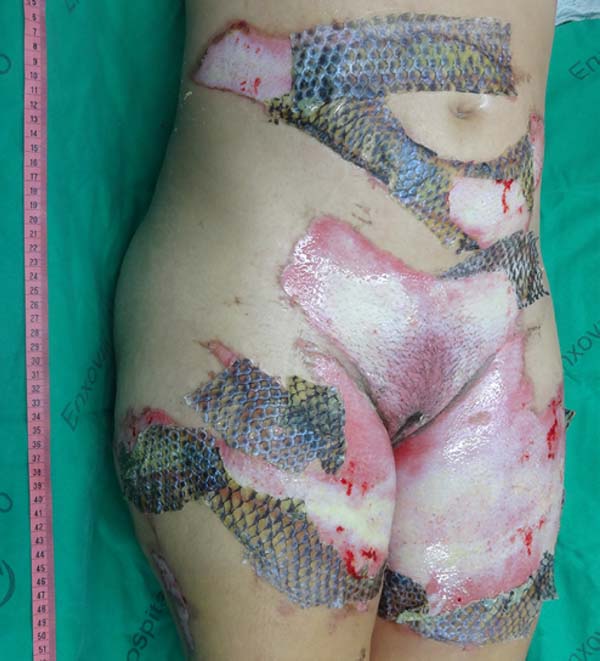
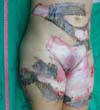
On the 14th day of treatment, after opening the dressing, it was observed that several of the pieces of tilapia skin previously adhered, now had a dry, hardened appearance and had started to come off. These pieces were removed by digitally separating the burn bed with the aid of petroleum jelly, exposing the underlying scarred skin (Figure 7). The rest of the tilapia skin was removed in the following 48 hours, and the patient was discharged, totaling 16 days of treatment.
DISCUSSION
In the search for new therapeutic alternatives for the treatment of burns, biocompatible or biological dressings have been highlighted. Since allografts are challenging to obtain and have low availability, xenografts can be a viable alternative due to their greater safety and reduced price7. Although frog skin has been used as a treatment for burns in Brazil8, it has never been registered by the Agência Nacional de Vigilância Sanitária (ANVISA) and is no longer in use. Therefore, the tilapia skin promises to be an innovative product, easy to apply, and highly available, which can become the first animal skin nationally studied and registered by ANVISA for use in the treatment of burns, in addition to be the first aquatic animal skin in the world used for this purpose.
With the use of standard treatments, it is expected about three weeks for complete healing of deep second degree burns9. Therefore, the 16-day period required for reepithelialization of this patient’s burn and the absence of side effects suggested the effectiveness of tilapia skin as a flexible and adherent xenograft, with no antigenicity and toxicity, and the ability to conserve moisture and avoid the entry of microorganisms, characteristics of an ideal dressing for burns10. Besides, this case report contributes to reducing the limitations concerning the anatomical areas appropriate for the application of tilapia skin, since, even with the need for skin replacement, good results were obtained, including in the inguinal and genital regions. The decrease in the number of dressing changes is an essential factor in reducing pain in these patients, decreasing teamwork and hospital costs.
REFERENCES
1. World Health Organization (WHO). Burns. Genebra: WHO; 2018 Mar; [acesso em 2019 mar 10]. Disponível em: http://www.who.int/news-room/fact-sheets/detail/burns
2. Oliveira M. Tilapia’s turn. Pesquisa FAPESP. 2016 Nov; [citado 2019 mar 10]; 249:66-71. Disponível em: http://revistapesquisa.fapesp.br/en/2017/05/17/tilapias-turn
3. Lima Júnior EM, Bandeira TJPG, Miranda MJB, Ferreira GE, Parente EA, Piccolo NS, et al. Characterization of the microbiota of the skin and oral cavity of Oreochromis niloticus. J Heal Biol Sci. 2016;4(3):193-7.
4. Alves APNN, Verde MEQL, Ferreira Júnior AEC, Silva PGB, Feitosa VP, Lima Júnior EM, et al. Avaliação microscópica, estudo histoquímico e análise de propriedades tensiométricas da pele de tilápia do Nilo. Rev Bras Queimaduras. 2015;14(3):203-10.
5. Alves APNN, Lima Júnior EM, Piccolo NS, Miranda MJB, Verde MEQL, Ferreira Júnior AEC, et al. Study of tensiometric properties, microbiological and collagen content in nile tilapia skin submitted to different sterilization methods. Cell Tissue Bank. 2018 Sep;19(3):373-82.
6. Lima Junior EM, Picollo NS, Miranda MJB, Ribeiro WLC, Alves APNN, Ferreira GE, et al. Uso da pele de tilápia (Oreochromis niloticus), como curativo biológ ico oclusivo, no tratamento de queimaduras. Rev Bras Queimaduras. 2017;16(1):10-7.
7. Hermans MHE. Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: is there a clinical difference?. Burns. 2014 May;40(3):408-15.
8. Piccolo N, Piccolo MS, Piccolo MTS. Uso de pele de rã como curativo biológico como substituto temporário da pele em queimaduras. Rev Bras Queimaduras. 2002;2:18-24.
9. Pan SC. Burn blister fluids in the neovascularization stage of burn wound healing: a comparison between superficial and deep partial-thickness burn wounds. Burns Trauma. 2013 Jun;1(1):27-31.
10. Chiu T, Burd A. “Xenograft” dressing in the treatment of burns. Clin Dermatol. 2005;23:419-23.
1. Instituto Dr. José Frota, Centro de Tratamento de Queimados, Fortaleza, CE, Brazil.
2. Universidade Federal do Ceará, Núcleo de Pesquisa e Desenvolvimento de Medicamentos,
Unidade de Farmacologia Clínica, Fortaleza, CE, Brazil.
Institution: Universidade Federal do Ceará, Núcleo de Pesquisa e Desenvolvimento de Medicamentos, Unidade de Farmacologia Clínica, Fortaleza, CE, Brazil.
COLLABORATIONS EMLJ
COLLABORATIONS Analysis and/or data interpretation, Conception and design study, Conceptualization, Data Curation, Final manuscript approval, Investigation, Methodology, Project Administration, Realization of operations and/or trials, Resources, Supervision, Writing - Original Draft Preparation, Writing - Review & Editing.
COLLABORATIONS MOMF
COLLABORATIONS Analysis and/or data interpretation, Conception and design study, Conceptualization, Data Curation, Final manuscript approval, Investigation, Methodology, Project Administration, Realization of operations and/or trials, Resources, Supervision, Writing - Original Draft Preparation, Writing - Review & Editing.
COLLABORATIONS BAC
COLLABORATIONS Analysis and/or data interpretation, Conceptualization, Data Curation, Final manuscript approval, Investigation, Realization of operations and/or trials, Validation, Visualization, Writing - Original Draft Preparation, Writing - Review & Editing.
COLLABORATIONS AMNU
COLLABORATIONS Analysis and/or data interpretation, Data Curation, Final manuscript approval, Investigation, Realization of operations and/or trials, Writing - Original Draft Preparation, Writing - Review & Editing.
COLLABORATIONS CBM
COLLABORATIONS Analysis and/or data interpretation, Data Curation, Final manuscript approval, Investigation, Realization of operations and/or trials, Writing - Original Draft Preparation, Writing - Review & Editing.
COLLABORATIONS MEAM
COLLABORATIONS Conception and design study, Conceptualization, Final manuscript approval, Methodology, Project Administration, Resources, Supervision, Visualization, Writing - Original Draft Preparation, Writing - Review & Editing.
COLLABORATIONS MBSR
COLLABORATIONS Conception and design study, Conceptualization, Final manuscript approval, Methodology, Project Administration, Resources, Supervision, Visualization, Writing - Original Draft Preparation, Writing - Review & Editing.
COLLABORATIONS FVF
COLLABORATIONS Conception and design study, Conceptualization, Data Curation, Final manuscript approval, Methodology, Project Administration, Supervision, Validation, Visualization, Writing - Original Draft Preparation, Writing - Review & Editing.
Corresponding author: Edmar Maciel Lima Júnior Instituto Dr. José Frota (IJF), Rua Barão do Rio Branco, 1816, Centro, Fortaleza, CE, Brazil. Zip Code: 60025-061 E-mail: edmarmaciel@gmail.com
Article received: March 12, 2019.
Article accepted: April 21, 2019.
Conflicts of interest: none.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter