

Original Article - Year 2020 - Volume 35 - Issue 2
Plastic surgery in a medium complexity hospital: prospective cohort with cost and results analysis of the treatment of skin tumors within the scope of the Unified Health System (SUS)
Cirurgia plástica em hospital de média complexidade: coorte prospectiva com análise de custos e dos resultados do tratamento de tumores cutâneos no âmbito do Sistema Único de Saúde (SUS)
ABSTRACT
Introduction: The Brazilian health system is organized into basic health units, medium complexity hospitals, and high complexity hospitals. The composition of medium complexity services is variable, with or without specialists such as the plastic surgeon. The objective of the present study is to analyze data on the treatment of patients with skin tumors by a plastic surgeon in a medium complexity hospital.
Methods: prospective cohort with analysis of epidemiological, demographic data, costs, results, complications, and degree of satisfaction.
Results: In nine months, 166 patients were treated, of whom 103 underwent surgery. The most common pathologies were: basal cell carcinomas, benign subcutaneous and cutaneous tumors, squamous cell carcinoma and melanoma, in decreasing order of appearance. Most of the injuries were treated with exeresis and suture surgery and in 29 patients, more complex reconstructions were required. The degree of resolution of cases was high, and only a patient was derived to a high complexity hospital. The degree of satisfaction with the treatment was also high. However, the estimated financial transfer of SUS, in the case of malignant tumors treatment, was approximately 25% less than it is in a high complexity hospital.
Conclusion: The role of the plastic surgeon in medium complexity hospitals can prevent diseases such as skin cancer from reaching high complexity hospitals in advanced stages. However, there would have to be adaptations in the government transfer to enable the routine performance of this professional in these institutions.
Keywords: Skin neoplasms; Unique Health System; Costs and cost analysis; Therapeutic interventions results evaluation; Plastic surgery.
RESUMO
Introdução: O sistema único de saúde brasileiro organiza-se basicamente em unidades básicas de saúde, hospitais de média e hospitais de alta complexidade. A composição dos serviços de média complexidade é variável, podendo contar ou não com especialistas como o cirurgião plástico. O objetivo do presente trabalho é o de descrever dados do tratamento dos pacientes com tumores cutâneos atendidos por um cirurgião plástico, em um hospital de média complexidade.
Métodos: Coorte prospectiva com análise de dados epidemiológicos demográficos, de custos, resultados, complicações e grau de satisfação.
Resultados: No período de 9 meses foram atendidos 166 pacientes dos quais 103 foram operados. As patologias mais comuns foram: carcinomas basocelulares, tumores benignos de subcutâneo e pele, carcinoma epidermóide e melanoma, em ordem decrescente de ocorrência. A maioria das lesões foi tratada com cirurgia de exérese e sutura. Reconstruções mais complexas foram necessárias em 29 pacientes. O grau de resolução dos casos foi alto, com apenas um paciente sendo encaminhado a hospital de alta complexidade. O grau de satisfação com o tratamento também foi elevado. Contudo, o repasse financeiro estimado do SUS, no caso do tratamento dos tumores malignos, foi em torno de 25% menor do que seria na alta complexidade.
Conclusão: A atuação do cirurgião plástico em hospitais de média complexidade pode impedir que doenças como o câncer de pele cheguem em estágios avançados em hospitais de alta complexidade, com alto grau de satisfação. Contudo, adaptações no repasse governamental precisariam ocorrer para viabilizar a atuação rotineira deste profissional nestas instituições.
Palavras-chave: Neoplasias cutâneas; Sistema Único de Saúde; Custos e análise de custo; Avaliação de resultado de intervenções terapêuticas; Cirurgia plástica
INTRODUCTION
The Unified Health System (SUS) is organized into basic health units and medium and high complexity establishments. The entrance door to the system is the basic health unit, which must solve the main problems with economic and straightforward measures and act in the prevention of the disease. Medium and high complexity hospitals, on the other hand, have specialized professionals and special technological resources1.
The division between medium and high complexity represents a great difficulty for SUS1 managers, and, unfortunately, their vision of this area is still fragmentary, with sets of procedures listed in the traditional “system procedure tables,” for outpatients or hospitals. This system is often a limiting factor that mainly penalizes medium-sized institutions that carry out many of the procedures and that, according to these tables, would be exclusive of high complexity.
The specialist professionals who work in these medium complexity hospitals experience that reality every day, as these places have more and more resources and specialists from multiple areas, with great potential for resolution, avoiding most transfers to institutions of high complexity already overcrowded. This situation happens not only because midsize hospitals have the structure and qualification to handle many of the so-called highly complex procedures, but also because high complexity cannot absorb all of these cases due to the lack of vacancies.
In the context of skin tumors, cared for in medium complexity hospitals, the above is repeated mainly in the case of malignant tumors. These are hospitals that have the resources to operate most tumors, including the most extensive malignant tumors, as long as they have a professional who specializes in plastic surgery or similar areas. These institutions have a surgical center, anesthetist, and even an Intensive Care Unit (ICU), which allows for numerous procedures. However, surgical codes in oncology are restricted to high complexity.
Non-melanoma skin tumors are the most frequent cancer in the population2,3, and the inclusion of medium hospitals in the care of these lesions could contribute to reducing the waiting time for referral to highly complex institutions. Unfortunately, in addition to not receiving resources for treatment according to the codes in oncology, these institutions do not always have professionals capable of treating these tumors in more advanced cases. Therefore, it is necessary to evaluate the cost and benefits of treating these injuries, as well as to discuss possible solutions for a better functioning of the system.
OBJECTIVE
The objective of the present study is to describe the experience of a plastic surgeon for nine months in the treatment of patients with skin tumors in a medium institution, not only to describe the epidemiology and results of the treatment of these injuries, but also discussing how to optimize the care of these patients and estimate the costs under the SUS.
METHODS
This one is a prospective cohort study carried out from January 2017 to September 2017 at the Getúlio Vargas Hospital in Sapucaia do Sul, Rio Grande do Sul. Data collection was performed in an Excel(TM) database, with data demographics such as sex, age, surgery data, operated area, the technique performed, pathological result, number of surgeries, among others. The financial transfer, according to SUS tables, was estimated for major surgeries performed in the operating room and only for malignant skin tumors. The degree of satisfaction was measured according to the Glasgow Benefit Inventory (GBI) scale, which was applied to part of the sample in patients contacted approximately two years after surgery. This questionnaire consists of 18 questions whose answers are rated from 1 to 5, which measure a global score and global, social, and health subpoints ranging from -100 (maximum negative impact) to +100 (maximum positive impact)4,5. Costs were estimated according to the codes used for each patient and based on the SUS website with the tables available online6. These last data were compared with the exclusive codes of high complexity that would be more appropriate for the case of each patient.
Inclusion criteria in the general cohort were all patients with benign and malignant skin and subcutaneous tumors who consulted during the study period. Although this outpatient clinic focuses on soft tissue tumors, patients with other pathologies such as dermatochalasis of lid, ectropion, breast hypertrophy, exposed orthopedic plates, and complex wounds were also treated, but were excluded from the present study. Data were analyzed in SPSS, version 20, IBMTM.
This work was presented to the Brazil Platform and approved by the Research Ethics Committee (CEP) number 5329, President Vargas Maternal and Child Hospital (HMIPV-RS), designated by the National Research Ethics Commission (CONEP), under the number Certificate of Presentation and Ethical Appreciation (CAAE) 16036719.2.0000.5329. The patients with photos published in this work approved their disclosure through a Free and Informed Consent Form (ICF). Patients whose data are reported here together, without the possibility of identification, were released from IC.
Four types of surgery regimens were used depending on the case to be treated: in an outpatient room with local anesthesia, in a surgical block with local anesthesia, in an operating room with sedation or an operating room with general anesthesia. The management of the patients was carried out following the “guidelines” for the removal of skin tumors7,8, and the reconstructions were performed according to the case and based on the current literature9-11, respecting the resources available in the Institution.
RESULTS
During the study period, 166 patients were treated, 63 of whom were excluded from the analysis because they were patients with pathologies other than skin and subcutaneous tumors, or patients who did not undergo surgery. One hundred three patients underwent surgery and who had complete data for analysis.
The average age of the operated patients was 60.1 years. The presence of associated comorbidities was found in 50.5% of the sample. The most frequent were: systemic arterial hypertension, diabetes, hypothyroidism, and depression, most of whom had more than one comorbidity at the same time. The average number of consultations was three per patient. Table 1 shows the demographic data for the cohort.
| Variable | N (%) or Mean and standard deviation |
|---|---|
| Gender | |
| Masculine | 45 (43.7) |
| Feminine | 58 (56.3) |
| Age | 60.12 (16.0)* |
| Presence of Comorbidities | 52 (50.5) |
| Multiple | 25 (24.3) |
| Hypertension | 9 (8.7) |
| Others | 18 (17.5) |
| Number of consultations per patient | |
| One | 16 (15.5) |
| Two | 32 (31.1) |
| Three | 25 (24.3) |
| Four | 15 (14.6) |
| Five or more | 15 (14.6) |
| Surgery Location | |
| Ambulatory | 53 (51.5) |
| Surgery Center | 50 (48.5) |
| Operated Area | |
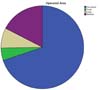
| Face | 72 (69.9) |
| Multiples areas | 18 (17.3) |
| Members | 8 (7.8) |
| Trunk | 5 (4.9) |
| Reconstruction technique | |
| Exeresis and suture | 74 (71.8) |
| Exeresis and flap | 21 (20.4) |
| Exeresis and graft | 8 (7.4) |
| Reconstruction (graft/flaps) | 29 (28.1) |
| Late reconstruction (post-AP) | 9 (8.7) |
| Anatomopathological Diagnosis | |
| Basal cell carcinoma | 46 (44.7) |
| Squamous cell carcinoma | 9 (8.7) |
| Melanoma | 3 (2.9) |
| Epidermal Cysts | 13 (12.6) |
| Others | 27 (26.2) |
| Margins (malignant tumors not melanoma) | 55 (53.4) |
| Free | 46 (83.6) |
| Comprometida | 9 (16.4) |
| Number of surgery per patient | |
| One | 87 (84.5) |
| Two | 11 (10.7) |
| Three | 3 (2.9) |
| Four | 2 (1.9) |
| Complications | 2 (1.9) |
* Data with normal distribution by the Shapiro-Wilk test represented by the mean of standard deviation.
The main areas of the body affected by the tumors were the face, followed by multiple areas as extremities and trunk (Figure 1). Basal cell carcinoma was the most commonly found tumor (44.7%), followed by benign skin and subcutaneous tumors, including nevi, epidermal cysts, and lipomas. Epidermoid tumors were found in 8.7% of cases, and three patients with melanomas underwent surgery. All of the latter were in the initial stage (less than 0.75 mm thick and without ulcerations), and only needed an increased margin, without the need for other interventions12,13. Taking into account the risk classification for nonmelanoma skin tumors of the NCCN (National Comprehensive Cancer Network) whose criteria are set out in Table 2 14, 38.7% were low risk, 55.5% high risk, and 5.8% already locally advanced.
| Low Risk ' | High Risk | |
|---|---|---|
| Location/Size | < 20mm in area L | > 20mm in area L |
| < 10mm in area M | > 10mm in area M | |
| < 0,6mm in area H | > 0,6mm in area H | |
| Margins | Well defined | Badly defined |
| History | Primary | Recurrents |
| Immunosuppression | No | Yes |
| Prior radiation | No | Yes |
| Anatomopathological diagnosis | Nodular/superficial BCC | BCC: moreaform, basal squamous, sclerodermiform, micronodular SCC: acantholytic, adenosquamous, desmoplastic, metaplastic |
Zone H: Central face, eyelids, eyebrow, nose, lips, chin, jaw, pre-auricular, post-auricular, ears, genitals, hands, feet; Zone L: Trunk, limbs (excluding nails, feet, hands and pre-tibial); Zone M: Cheeks, forehead, scalp, neck and pre-tibial; BCC: basal cell carcinoma; SCC: Squamous cell carcinoma.
Nine patients with squamous cell and basal cell tumors had margins described as compromised or small. Of these, all were expanded, except one who refused to expand the margins and another who decided to observe and did not return for the expansion. Of the seven operated patients, two had residual carcinoma in the surgical sample, and only one remained with a compromised margin until reconstruction, losing the graft. The latter was the only patient who had to be referred to a high complexity center because he was a patient with squamous carcinoma little differentiated from the temporal region, already with an invasion of the parotid gland, locally advanced and with a poor prognosis. He was subsequently operated in conjunction with the head and neck surgeon, who performed a partial parotidectomy and a neck dissection. The patient was referred to radiotherapy after resection.
More complex reconstructions with grafts or flaps were necessary in only 28% of the total number of operated patients, with 31% of these patients opting for late reconstruction, leaving patients with a local bandage until pathological diagnosis (PA) definitive, followed by reconstruction around two weeks after the first surgery in the case of free margins.
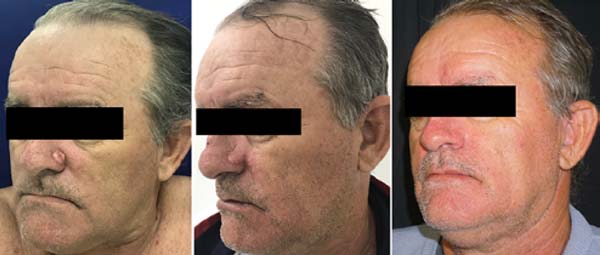
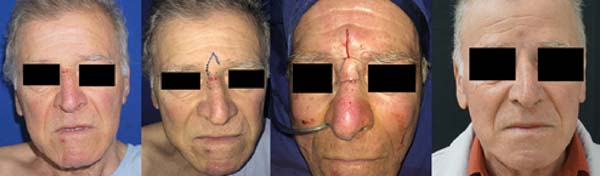
Twenty-one flaps were made that included the Limberg technique, bilobed, axial flap, glabellar flap (Rieger), nasogenian, and triple rhomboid flap of scalp. Figures 2 to 4 show results for some of these more complex reconstruction cases.

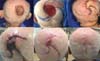
Complications occurred in 16.5% of patients, which included 5 cases of wound infection, one hematoma, one patient with persistent pain that remitted after three months, one suture dehiscence with fat necrosis, two partial flap necrosis, two scar hypertrophies, one partial graft loss, three trapdoor deformities that improved in 6 months, one temporary paralysis of the temporal branch of the facial due to the local anesthetic and a definitive paralysis of the temporal and zygomatic branch of the facial compromised by the tumor ( patient who performed the parotidectomy described above). Figure 5 shows a case of a patient with partial flap necrosis with proper final resolution with dressings, which shows a good result even in the case of complications.
Twenty patients were contacted to answer a questionnaire to calculate the GBI scale, 3 of whom had died, and nine did not answer the phone or did not attend the day of the interview, leaving only 8 to answer the questionnaire. In these, the improvement was shown in the four subdomains of the scale, with an average of 53.8 points on the general scale, 75 points on the general subscale, 4.1 on the social subscale, and 18.75 on the health subscale.
Taking into account the transfers according to the SUS table for surgery and only the patients operated on in the operating room for malignant tumors, this resulted in 37 patients for the estimation. In these patients, the estimated cost for SUS was 349.25 reais per patient, 253.38 reais per surgery. If it were possible to use cancer codes that are only authorized for high complexity, but that would be more suitable for the surgeries performed, the transfer could be an average of 470.94 per patient and 341.66 per surgery, that is, about 25% bigger. Furthermore, the standard procedures performed in this cohort of patients, such as the removal of multiple malignant skin tumors, the removal of lip and pinna tumors, do not find equivalent codes in the table of average complexity, and the code used in these cases. It was much lower in terms of transfer rates compared to those available in high complexity. Fortunately, in the case of the graft and flap codes, the transfer is smaller, but less impactful, that is, 10% less than the graft and flap codes after the oncological excision of highly complex tables.
DISCUSSION
Nonmelanoma skin tumors are the most frequent neoplasty; in the USA, its diagnosis is higher than the diagnosis of all cancers combined2. In the cohort presented in this study, basal cell carcinoma was the most commonly reported lesion, even surpassing benign skin and subcutaneous tumors. The most affected place was the face, followed by the extremities. The authors who have studied facial tumors15 and basal cell tumors (BCC) 5 have found similar results to those shown here16,17. In a recent review of keratinocytic skin cancers (nonmelanoma), it is noted that their incidence and, therefore, the costs of this disease have increased worldwide, with an estimated risk of 20-30% of development during life in white patients, according to US estimates7,8,18.
These tumors can be divided into high and low risk, according to the criteria proposed by the NCCN19 shown in Table 2, translated by Hughley et al., In 201814. It should be noted that in this cohort, according to these specifications, the majority of patients (61.3%) already had high-risk or locally advanced squamous cell and basal cell carcinomas.
In these cases of facial tumors, flaps are preferred over grafts because they have better aesthetic results16. As in our case series, the most affected area was the face, in the case of reconstructions, flaps were preferred, with 21 flaps versus eight grafts performed, with only 2 of these graft cases used on the face. In 9 cases, in which flap reconstructions were proposed, late reconstruction was chosen only after pathology, demonstrating that disease-free margins decrease the risk of reconstruction loss, as recommended in the literature7,8,14,19. According to the guidelines for the treatment of skin tumors, the best indication for many cases in this cohort would be Mohs micrographic surgery since they are lesions with various high-risk criteria, according to NCCN14. However, this technique is not available in the institution, opting for standard resection and late reconstruction in the case of the need for a significant mobilization of tissues for reconstruction.
Regarding the analysis of costs, results, and degree of patient satisfaction, it is important to clarify from which perspective this analysis is carried out20. In the case of this cohort, from the hospital’s perspective, there is a particular financial loss for the institution regarding the care of these patients; however, for SUS, there is a benefit in the treatment of these injuries at a medium level, since the transfer is less than that performed at high complexity and also because the access is fast. This study compares the transfer of SUS between medium and high complexity, but does not estimate whether this transfer covers the real hospital costs with the treatment of these injuries. In this context, Bócoli et al., In 201321, made this comparison, demonstrating that the internal expenditure on the treatment of these injuries is higher than that transferred by SUS. However, in this study, the author does not specify if the estimation was made in a medium or high complexity hospital, he only mentions that it was in a university hospital, probably a high complexity one. Therefore, considering the findings of Bócoli et al., In 201321, the loss of the institution of medium complexity is probably even more significant than that shown in this study in highly complex hospitals.
Regarding the results, there was a low rate of complications, especially considering that it is a cohort with several tumors with a high risk of recurrence and because it is a population with a high average age and the presence of comorbidities in 50.5% of the sample. Besides, according to the questionnaire on the degree of satisfaction that was applied to part of the patients, there is an improvement in all the domains of the questionnaire, mainly in the general scores and the general scores. Furthermore, regarding resolution capacity, we highlight that only one patient could not be treated with medium complexity, being referred to a highly complex hospital. Therefore, from a population perspective, the benefits are many.
CONCLUSION
Because of the preceding and considering that nonmelanoma skin cancer is a disease that is increasing worldwide and in Brazil, it is concluded that this disease has and will continue to have a critical impact on the Unified Health System that should seek alternatives for allowing access to these patients for effective treatment, in this context, the inclusion of medium-sized hospitals in the care of these patients seems to be a great strategy to optimize the operation of the system; however, the feasibility of this routine action at this level of health care should undergo a review in the SUS procedure tables, which should be updated, since in practice several procedures of exclusivity of high complexity are already performed in medium complexity, through replacement codes that mostly do not contemplate the real complexity of the procedures performed, with values received 25% lower.
The presence of qualified professionals to adequately treat this problem, such as the plastic surgeon at the medium level of complexity, can significantly contribute to resolving this high-incidence disease without referral to high complexity. According to the cohort presented here, the degree of resolution of cases of medium complexity was excellent, and the routine inclusion of medium-sized institutions in the treatment of skin cancers would collaborate with better patient care and better SUS functioning.
REFERENCES
1. Ministério da Saúde (BR). Conselho Nacional de Secretários de Saúde (CONASS). Assistência de média e alta complexidade no SUS. Brasília (DF): CONASS; 2007.
2. Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018 Jul;379(4):363-74.
3. Brandt MG, Moore CC. Nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2019 Feb;27(1):1-13.
4. Kotzampasakis D, Piniara A, Themelis S, Kotzampasakis S, Gabriel E, Maroudias N, et al. Quality of life of patients who underwent aesthetic rhinoplasty: 100 cases assessed with the Glascow Benefit Inventory. Laryngoscope. 2017 Sep;127(9):2017-25.
5. Hornos A. Correção de orelha de abano por técnica combinada: análise de resultados e alteração da qualidade de vida. Rev Bras Cir Plást. 2001 Jan;28(3):406-15.
6. Ministério da Saúde (BR). Sistema de Gerenciamento da Tabela de Procedimentos, Medicamentos e OPME do SUS (SIGTAP). Tabela unificada [Internet]. Brasília (DF): SIGTAP, 2019; Mai;1. Available from: http://sigtap.datasus.gov.br/Tabela-unificada/app/sec/inicio.jsp
7. Kim JYS, Kozlow JH, Mittal B, Moyer J, Olencki T, Rodgers P. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018 Mar;78(3):540-59.
8. Alam M, Armstrong A, Baum C, Bordeaux JS, Brown M, Busam KJ, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018 Mar;78(3):560-78.
9. Mélega JM. Cirurgia Plástica - Fundamentos e Arte. Rio de Janeiro: MEDSI; 2002.
10. Jackson IT. Retalhos locais na reconstrução de cabeça e pescoço. Rio de Janeiro: DiLivros; 2002.
11. Baker SR. Baker: local flaps in facial reconstruction. 2nd ed. Philadelphia, PA: Elsevier Health Sciences; 2007.
12. Knackstedt T, Knackstedt RW, Couto R, Gastman B. Malignant melanoma: diagnostic and management update. Plast Reconstr Surg. 2018 Aug;142(2):202e-16e.
13. Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet. 2018 Sep;392(10151):971-84.
14. Hughley BB, Schmalbach CE. Cutaneous head and neck malignancies in the elderly. Clin Geriatr Med. 2018 May;34(2):245-58.
15. Laitano FF, Teixeira LF, Siqueira EJ, Alvarez GS, Martins PDE, Oliveira MP. Use of skin flaps for nasal reconstruction after neoplastic resection. Rev Bras Cir Plást. 2012 Jun;27(2):217-22.
16. Broetto J, Freitas JOG, Sperli AE, Soh SW, Richter CA, Toni RA. Surgical treatment of basal and squamous cell carcinomas: experience of the Plastic Surgery Services of Hospital Ipiranga. Rev Bras Cir Plást. 2012 Dec;27(4):527-30.
17. Souza CFD, Thomé EP, Menegotto PF, Schmitt JV, Shibue JRT, Tarlé RG. Topografia do carcinoma basocelular e suas correlações com o gênero, a idade e o padrão histológico: um estudo retrospectivo de 1.042 lesões. An Bras Dermatol. 2011 Apr;86(2):272-7.
18. Cameron MC, Lee E, Hibler BP, Barker CA, Mori S, Cordova M, et al. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-17.
19. Bariani RL, Nahas FX, Barbosa MVJ, Farah AB, Ferreira LM. Basal cell carcinoma: an updated epidemiological and therapeutically profile of an urban population. Acta Cir Bras. 2006 Mar/Apr;21(2):66-73.
20. Blank MM, Chen L, Papageorge M, Driscoll D, Graham R, Chatterjee A. The underreporting of cost perspective in cost-analysis research: A systematic review of the plastic surgery literature. J Plast Reconstr Aesthet Surg. 2018 Mar;71(3):366-76.
21. Bócoli KH, Veiga DF, Cabral IV, Carvalho MP, Novo NF, Veiga Filho J, et al. Tratamento cirúrgico de carcinomas cutâneos pelo Sistema Único de Saúde: análise de custos. Rev Col Bras Cir. 2013 Dec;40(6):449-52.
1. Hospital Ernesto Dornelles, Porto Alegre, RS, Brazil.
2. Hospital de Pronto Socorro de Porto Alegre, Porto Alegre, RS, Brazil.
3. Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brazil.
Corresponding author: Daniele Walter Duarte, Rua General Caldwell 969, Bairro Menino Deus, Porto Alegre, RS. Brazil. Zip Code: 90130-051. E-mail: daniwalterduarte@gmail.com
Article received: December 21, 2019.
Article accepted: February 22, 2020.
Conflicts of interest: none.










 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter