

Original Article - Year 2020 - Volume 35 -
Microbiological profile and antimicrobial resistance profile of patients admitted to the Burn Unit of Hospital Geral "José Pangella" in Vila Penteado in Vila Penteado, Brazil
Perfil microbiológico e de resistência aos antimicrobianos dos pacientes internados na Unidade de Queimaduras do Hospital Geral "José Pangella" de Vila Penteado
ABSTRACT
Introduction: Burns are responsible for about 180,000 deaths per year worldwide and about 1,000,000 accidents, more than 100,000 hospital admissions and 2,500 deaths per year in Brazil. Among the causes of morbidity and mortality of burn patients, infections stand out. Knowledge of the microbiological profile and appropriate treatment of infection cases impact on the decrease in morbidity and mortality rates. The Objetive is to analyze the microbiological profile and antimicrobial resistance profile of patients admitted to the Burn Unit of the General Hospital "José Pangella" of Vila Penteado from 2011 to 2018.
Methods: This is a retrospective study and surveyed all microbiological examinations of patients hospitalized for burns at the "José Pangella" Burns Unit of Vila Penteado General Hospital, located in the city of São Paulo, from January 2011 until the end of December 2018.
Results: 495 microorganisms were isolated, being 436 bacteria (88,080%) and 59 fungi (11,919%). Among the samples analyzed, the highest prevalence was Staphylococcus sp., followed by Pseudomonas sp., Klebsiella sp., Candida sp. and Acinetobacter sp.
Conclusion: Handling burn patients remains a major challenge for burn treatment centers. Identifying the pathogens responsible for patients infections may result in optimal treatment, with an effective antibiotic choice and reducing the morbidity and mortality of these patients, as well as significantly reducing hospitalization time and costs.
Keywords: Burn units; Burns; Microbial sensitivity tests; Indicators of morbidity and mortality; Bacterial infections and mycoses; Bacterial infections.
RESUMO
Introdução: As queimaduras são responsáveis por cerca de 180.000 mortes por ano no mundo e cerca de 1.000.000 de acidentes, mais de 100.000 internações hospitalares e 2.500 mortes por ano no Brasil. Dentre as causas de morbidade e mortalidade do paciente queimado, destacam-se as infecções. O conhecimento do perfil microbiológico e o adequado tratamento dos casos de infecção impactam na diminuição nas taxas de morbimortalidade. O objetivo é analisar o perfil microbiológico e de resistência aos antimicrobianos dos pacientes internados na Unidade de Queimaduras do Hospital Geral "José Pangella" de Vila Penteado, durante o período de 2011 a 2018.
Métodos: O estudo é retrospectivo e levantou todos os exames microbiológicos dos pacientes internados por queimaduras na Unidade de Queimaduras do Hospital Geral "José Pangella" de Vila Penteado, localizado na cidade de São Paulo, durante o período de janeiro de 2011 até o final de dezembro de 2018.
Resultados: Foram isolados 495 microrganismos, sendo 436 bactérias (88,080%) e 59 fungos (11,919%). Entre as amostras analisadas, a maior prevalência foi do Staphylococcus sp., seguido por Pseudomonas sp. e Klebsiella sp., destacando-se, ainda, Candida sp. e Acinetobacter sp.
Conclusão: O manuseio dos pacientes vítimas de queimaduras continua sendo um grande desafio para os centros de tratamento de queimaduras. Identificar os patógenos responsáveis pelas infecções dos pacientes pode acarretar em uma otimização do tratamento, com a escolha de um antibiótico eficaz, e, dessa forma, acarretar na redução da morbimortalidade desses pacientes, além de diminuir tempo de internação e custos utilizados de maneira significativa.
Palavras-chave: Unidades de queimados; Queimaduras; Testes de sensibilidade microbiana; Indicadores de morbimortalidade; Infecções bacterianas e Micoses; Infecções bacterianas
INTRODUCTION
Burns are considered a public health problem due to their high prevalence1. They are responsible for around 180,000 deaths per year worldwide2 and are classified as the fourth most common type of trauma, second only to traffic accidents, falls, and interpersonal violence 3. They mainly affect low-income and developing countries, where mortality is up to 11 times higher than in developed countries3. The United States has the highest burn victim mortality rate among industrialized countries. In Brazil, according to the Sociedade Brasileira de Queimaduras (SBQ Brazilian Burns Society), burns are responsible for approximately 1,000,000 accidents, more than 100,000 hospitalizations, and 2,500 deaths per year4-7.
Among the causes of morbidity and mortality of burned patients, infections stand out (the leading cause of mortality in Brazil and worldwide)8. Therefore, 75% of deaths in patients with 40% or more surface area body burns are due to secondary infections9.
It is inferred, therefore, that knowledge of the microbiological profile responsible for infections in this group of patients and the choice of the most effective antibiotics in their treatment, would result in a decrease in morbidity and mortality rates and a shorter hospital stay and a lower number of interventions, thus resulting in a reduction in public spending.
OBJECTIVE
To analyze the microbiological and antimicrobial resistance profile of patients admitted to the Burns Unit of the General Hospital “José Pangella” in Vila Penteado, during the period from 2011 to 2018.
METHODS
The study was submitted to the Ethics and Research Committee of the General Hospital of Grajaú - Associação Congregação de Santa Catarina (Opinion Number: 3,635,831) and, after its approval (CAAE 23032719.9.0000.5447), we were given access to the clinical records of patients admitted to the Burns Unit.
This one is a retrospective, cross-sectional study, through the analysis of all microbiological exams of patients hospitalized for burns at the Burn Unit of the General Hospital “José Pangella” in Vila Penteado, located in the city of São Paulo, from January 2011 to the end of December 2018.
All patients were submitted to the Free and Informed Consent Form (FICF) and agreed with it.
Patients of both sexes, aged between 8 and 91 years, were evaluated. The burned body surface varied according to each patient.
The patients were admitted to the burn unit in the period analyzed, according to the following admission criteria: partial-thickness burns> 10% of the burned body surface (BBS); burns in particular regions (face, hands, feet, genitals, perineum, neck, or large joints); partial or full-thickness deep burns at any age; circumferential burns at any age; electrical, chemical burns, suspected of inhalation injury, associated with trauma or concomitant disease; In addition to critically ill patients who needed intensive care10.
Four hundred twenty-six culture exams of a total of 250 patients admitted to the unit in the specified period were analyzed. Such samples were collected during the entire period of hospitalization of the patient, both at the time of admission and at times with an infectious clinic.
Cultures of blood samples (247 samples), urine (31 samples), tracheal discharge (2 samples), vaginal discharge (1 sample), anal swab (12 samples), axillary swab (2 samples), oral swab (1), nasal swab (2 samples), discharge from the lesion (77 samples) and catheter tip (51 samples) were analyzed. All the samples were collected following the collection rules of the Hospital Infection Control Center (CCIH) of the hospital, so that there was no contamination, and they were sent and processed by the laboratory of the Associação Fundo de Incentivo à Pesquisa (AFIP Incentive Association for Research), located in the city of Sao Paulo The collected samples were seeded in specific culture media (blood agar, chocolate agar, and MacConkey agar) and were identified after growth.
In addition to counting the microorganisms present, their sensitivity to the antibiotics currently used in the corresponding groups was also verified, reading their respective antibiograms. These antibiograms, as well as the cultures, were also analyzed and released by the laboratory of the Associação Fundo de Incentivo à Pesquisa (AFIP), located in the city of São Paulo.
RESULTS
Four hundred twenty-six microbiological examinations of 250 different patients who were admitted to the Burned Unit of the General Hospital “José Pangella” in Vila Penteado from January 2011 to the end of December 2018 were evaluated. From these tests, 495 microorganisms were isolated, 436 bacteria (88,080%) and 59 fungi (11,919%) (Table 1).
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Patients | 6 | 30 | 43 | 14 | 17 | 36 | 65 | 39 | 250 |
| Cultures | 7 | 61 | 88 | 17 | 30 | 51 | 105 | 67 | 426 |
| Microorganisms | 7 | 67 | 97 | 17 | 30 | 62 | 130 | 85 | 495 |
| Bacterias | 5 | 58 | 73 | 17 | 28 | 60 | 122 | 73 | 436 |
| Fungi | 2 | 9 | 24 | 0 | 2 | 2 | 8 | 12 | 59 |
Of these 426 microbiology exams, they were evaluated 247 blood culture samples (57.891%), 31 urine cultures (7.276%), 51 catheter tip cultures (11.971%), 2 tracheal secretion samples (0.449%), 1 of vaginal secretion (0.234%), 12 samples of anal swab (2.816%), 2 samples of axillary swab (0.469%), 1 oral swab (0.234%), 2 nasal swab (0.469%) and 77 samples of secretion from burned injuries (18.075%).
From these exams, we can highlight blood cultures, catheter tip samples (associated with another positive blood culture) and urine cultures (total of 355 samples or 77.138% of the total) as representative of systemic infection with laboratory microbiological evidence, since they represented circulation of the microorganisms and were collected at moments compatible with the patient’s infectious clinical condition. These samples were called, by the author, clinically relevant to the study.
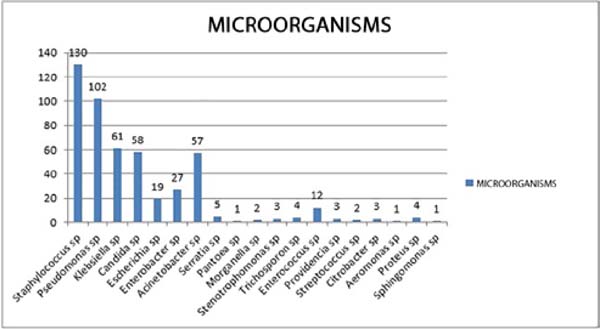
Among the samples analyzed, the highest prevalence was Staphylococcus sp. (130 cases or 26.262%), followed by Pseudomonas sp. (102 cases or 20.606%) and Klebsiella sp. (61 cases or 12.323%), with Candida sp. (58 cases or 11.717%) and Acinetobacter sp. (57 cases or 11.515%) (Figure 1).
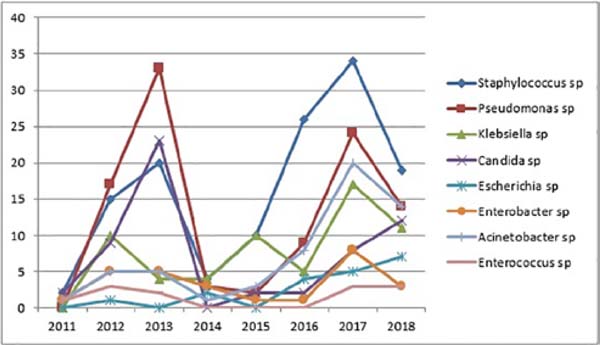
There was also an increase in the positivity of the samples in recent years, with Staphylococcus sp., Pseudomonas sp., Acinetobacter sp., and Klebsiella sp. (Figure 2).
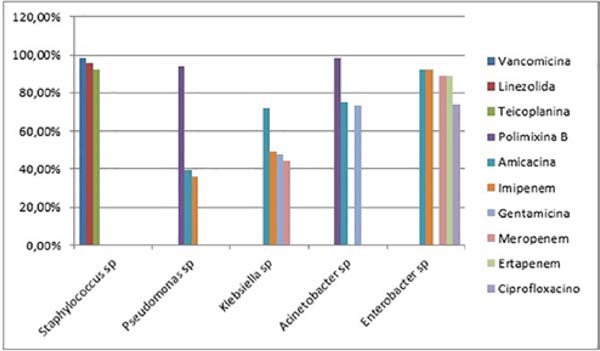
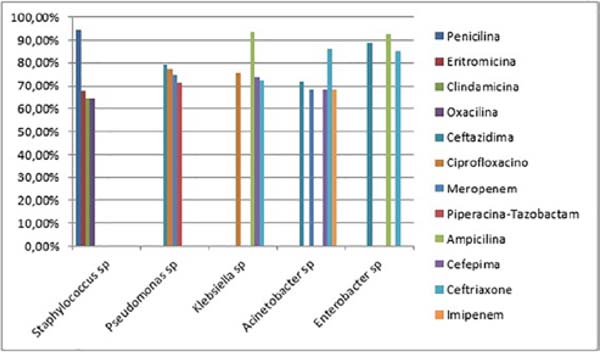
The antibiotic sensitivity profiles of the five most common microorganisms in the study (Staphylococcus sp., Pseudomonas sp., Klebsiella sp., Acinetobacter sp. and Enterobacter sp.) were also analyzed, disregarding Candida sp., since antifungigram is not routinely performed, as the mutation profile for resistance to yeast antifungals is low (28). The strains of Staphylococcus sp. were sensitive to Vancomycin (128 out of 130 microbiological tests or 98.461%), Linezolid (124 out of 130 or 95.384%) and Teicoplanin (120 out of 130 or 92.307%), while being resistant to Penicillin (123 out of 130 or 94.615%), Erythromycin (88 out of 130 or 67.692%) and Clindamycin and Oxacillin (84 out of 130 or 64.615%). Pseudomonas sp. was sensitive to Polymyxin B (96 in 102 or 94,117%), Amikacin (40 in 102 or 39,215%) and Imipenem (37 in 102 or 36,274%), while being resistant to Ceftazidime (81 in 102 or 79,411%), Ciprofloxacin (79 in 102 or 77,450%), Meropenem (76 in 102 or 74,509%) and Piperazine-Tazobactam (73 in 102 or 71,568%). Klebsiella sp. was sensitive to Amikacin (44 in 61 or 72.131%), Imipenem (30 in 61 or 49.180%), Gentamicin (29 in 61 or 47.540%) and Meropenem (27 in 61 or 44.262%), while being resistant to Ampicillin (57 in 61 or 93.442%), Ciprofloxacin 46 in 61 or 75.409%), Cefepime (45 in 61 or 73.770%) and Ceftriaxone (44 in 61 or 72.131%). Acinetobacter sp. was sensitive to Polymyxin B (56 in 57 or 98.245%), Amikacin (43 in 57 or 75.438%) and Gentamicin (42 in 57 or 73.684%), while being resistant to Ceftriaxone (49 in 57 or 85.964%), Ceftazidime (41 out of 57 or 71.929%) and Cefepime, Imipenem and Meropenem (39 out of 57 or 68.421%). Finally, Enterobacter sp. was sensitive to Amikacin and Imipenem (25 in 27 or 92.592%), Ertapenem and Meropenem (24 in 27 or 88.888%) and Ciprofloxacin (20 in 27 or 74.074%), while being resistant to Ampicillin (25 in 27 or 92.592%) %), Ceftazidime (24 out of 27 or 88.888%) and Ceftriaxone (23 out of 27 or 85.185%) (Figures 3 and 4).
DISCUSSION
The infection of the burn patient remains a significant cause of morbidity and mortality in this group of patients despite having decreased in incidence in recent years due to improvements in diagnosis and treatment. It mostly affects the male population with 63% of cases11 and, around 50% of patients with the burned body surface, 20% develop sepsis12, while 75% of deaths in patients with 40% or more of burned body surface are due to secondary infections9. Other literatures point out infections as responsible for about 75% of all deaths in this group13-15, preferentially affecting the extremes of age groups, such as children (mainly 0-10 years old) and the elderly13,16,17. Some studies mention that burns in the child population represent up to 50% of all severe burns, in addition to the population up to 5 years old, representing 50-80% of all childhood burns1.
According to Coutinho et al., 201518, the average body surface burned in 171 patients admitted to the ICU was 28%.
In Brazil, data from the Ministry of Health show that spending on burn victims can reach up to one million reais per month19, with daily expenses of US $ 1,000 per day20 for non-fatal cases and more than R $ 1,620.00 for those who die21,22.
Despite being the fourth most common type of trauma, behind traffic accidents, falls, and interpersonal violence3, burns have the third place in accidental deaths in the world21, hence its great importance in public health worldwide. Its leading cause of hospitalization in adults is fire and flammable burns and scalds in pediatric patients1,10. According to the National Burn Information Exchange (1996), 60% of accidents happen in the home environment. Luiz Philipe Molina Vana, plastic surgeon and president of SBQ, says that this figure rises to 77%.
The risk of the burned patient contracting an infection varies according to the extent and depth of the injury14,23. These lesions, to a greater or lesser degree, are responsible for breaking the protective barrier of the skin, which facilitates the entry of microorganisms, in addition to the immunological depression caused in these patients, the formation of necrosis as a favorable environment for bacterial proliferation, of the various invasive procedures, the extended hospital stay of these patients, the gastrointestinal bacterial translocation, among others13. There is also vascular obstruction caused by thermal injury, which hinders the arrival of both antimicrobials and components of the immune system to the burned area15.
Contaminated wounds usually present phlogistic characteristics, such as hyperemia, heat, and discharge of secretion, in addition to, in cases of bacteremia, dysthermias and leukocytosis. Necrosis is a crucial culture medium for the growth of opportunistic microorganisms and needs to be removed as soon as possible. In the first 48 hours, the wounds are already colonized by gram-positive bacteria, which can be reduced with the use of topical antimicrobials. After about 5 to 7 days, however, they are colonized by gram-negative bacteria, of hospital origin or origin of the gastrointestinal or respiratory tracts24,25, which can have severe consequences for the patient, such as serious infections and increased morbidity and mortality.
According to Nasser et al., 200326, in the first week of hospitalization, gram-negative bacteria were predominant (55.7%) against gram-positive bacteria (40.3%), whereas, in the second week, this predominance of gram-negatives becomes even more evident (72.7% x 22.7%). Among bacterial pathogens, we must highlight the microorganisms that potentially cause serious infections, such as the gram-positive methicillin-resistant Staphylococcus aureus (MRSA) and the gram-negative Pseudomonas aeruginosa, requiring broad-spectrum antibiotic coverage. Its use on a large scale, however, favors the growth of fungal microorganisms, such as Candida, Aspergillus, and Mucor9.
Staphylococcus aureus, the most prevalent pathogen in wounds and blood cultures after the advent of Penicillin, has a mortality rate of up to 30% and can reach 45% when it comes to MRSA. The group A Beta Hemolytic Streptococcus, group A, the primary pathogen present in burn wounds before the development of Penicillin, stands out as a gram-positive27,28.
Of the gram-negatives, P. aeruginosa (most prevalent), Acinetobacter baumanniie, and Enterococcus spp stand out9,15,29.
The importance of these pathogens lies, in addition to their higher virulence, in the great capacity to develop resistance to the antibiotic treatments currently used. The use of broad-spectrum drugs should be used carefully to try to avoid the spread of these pathogens, which corroborates the importance of research related to microbiological profiles. Studies show that, of the patients infected with Acinetobacter baumanniie, 46% develop bloodstream infection and, of these, 38% end up dying30, showing the high virulence of the microorganism. Severe patients in the ICU or on mechanical ventilation for more than 24 hours are more likely to develop fungal infections, such as C. tropicalis, C. parapsilosis, C. krusei, and C. glabrata31. Thus, the knowledge of the profile of the most common microorganisms in each Burn Unit is essential to restrict the proliferation of these resistant pathogens.
The present study, as well as in the literature, demonstrates the prevalence of Staphylococcus sp. (26.262%), Pseudomonas sp. (20.606%) and Acinetobacter sp. (11.515%), in addition to highlighting the importance of others, such as Klebsiella sp. (12.323%) and Candida sp. (11.717%). It also shows a predominance of gram-negative (73.737%) over gram-positive (26.262%)9.
CONCLUSION
The handling of burn victims remains a significant challenge for burn treatment centers. Identifying the pathogens responsible for infections, as well as the appropriate choice of antibiotic therapy, can lead to an optimization of treatment and, thus, reduce the morbidity and mortality of these patients.
The rationalization of antimicrobial therapy is a mainstay of antibiotic administration programs and is associated with fewer side effects and lesser appearance of resistant microorganisms, in addition to significantly reducing hospital stay and costs.
Besides, it is observed that the number of positive cultures and infections remain high in the population studied, corroborating the importance of studying microbiological profiles.
REFERENCES
1. Medeiros ACS, Albuquerque BCH, Mignoni ISP, Pereima MJL, Baungratz MM, Feijó RS. Análise das causas de morte em uma unidade de queimados do Hospital Infantil Joana de Gusmão (HIJG), de janeiro de 1991 a dezembro de 2012. Rev Bras Queimaduras. 2013;12(3):153-8.
2. World Health Organization (WHO). Burns. Geneva: WHO; 2018; [acesso em 2019 agosto 16]. Disponível em: https://www.who.int/en/news-room/fact-sheets/detail/burns
3. Agbenorku P, Agbenorku M, Fiifi-Yankson PK. Pediatric burns mortality risk factors in a developing country's tertiary burns intensive care unit. Int J Burns Trauma. 2013 Jul;3(3):151-8.
4. Souza AA, Mattar CA, Almeida PC, Faiwichow L, Fernandes FS, Enéas Neto CA, et al. Epidemiological profile of patients admitted at Burn Unit Servidor Público Estadual de São Paulo Hospital. Rev Bras Queimaduras. 2009;8(3):87-90.
5. Henrique DM, Silva LD, Costa ACR, Rezende APMB, Santos JAS, Menezes MM, et al. Controle de infecção no centro de tratamento de queimados: revisão de literatura. Rev Bras Queimaduras. 2013;12(4):230-4.
6. Arruda FCF. Comparação de escores de gravidade para previsão de mortalidade e tempo de internação em unidade de queimados. Rev Bras Queimaduras. 2017;16(3):142-9.
7. Ministério da Saúde (BR). Queimados. Brasília (DF): Ministério da Saúde; 2017; [acesso em 2019 agosto 16]. Disponível em: http://www.saude.gov.br/component/content/article/842-queimados/40990-queimados
8. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006 Apr;19(2):403-34.
9. Oliveira FL, Serra MCVF. Infecções em queimaduras: revisão. Rev Bras Queimaduras. 2011;10(3):96-9.
10. Neligan PC. Cirurgia Plástica - Extremidade Inferior, Tronco e Queimaduras. Amsterdã: Elsevier Editora Ltda.; 2015. vol. 4.
11. Pereira NCS, Paixão GM. Características de pacientes internados no centro de tratamento de queimados no estado do Pará. Rev Bras Queimaduras. 2017;16(2):106-10.
12. Sala LGP, Lima NL, Simioni PU, Ugrinovich LA. Principais patógenos envolvidos em casos de sepse em pacientes queimados: uma revisão de literatura. Rev Bras Queimaduras. 2016;15(3):164-168.
13. Macedo JL, Rosa SC, Castro C. Sepsis in burned patients. Rev Soc Bras Med Trop. 2003;36(6):647-52.
14. Sodré CNS, Serra MCVF, Rios JAS, Cortorreal CG, Maciera L, Morais EN. Perfil de infecção em pacientes vítimas de queimadura no Hospital Federal do Andaraí. Rev Bras Queimaduras. 2015;14(2):109-12.
15. Henrique DM, Silva LD, Costa ACR, Rezende APMB, Santos JAS, Menezes MM, et al. Controle de infecção no centro de tratamento de queimados: revisão de literatura. Rev Bras Queimaduras. 2013;12(4):230-4.
16. National Safe Kids Campaign (NSKC). Burn fact sheet. Washington, DC: NSKC; 2002.
17. Pruitt Junior BA, Goodwin CW, Mason Junior AD. Epidemiological, demographic and outcome characteristics of burn injury. In: Herndon DN, ed. Total burn care. London: Saunders; 2002. p. 16-30.
18. Coutinho JGV, Anami V, Alves TDO, Rossatto PA, Martins JIS, Sanches LN, et al. Estudo de incidência de sepse e fatores prognósticos em pacientes queimados. Rev Bras Queimaduras. 2015;14(3):193-7.
19. Rossi LA, Ferreira E, Costa ECFB, Bergamasco EC, Camargo C. Prevenção de queimaduras: percepção de pacientes e de seus familiares. Rev Latino-Am Enferm. 2003;11(1):36-42.
20. Teodoro AL, Paiva VS. Perfil epidemiológico de pacientes queimados admitidos em um serviço terciário de Caxias do Sul - RS. Rev Bras Queimaduras. 2013;12(2):108-11.
21. Rempel LCT, Tizzot MRPA, Vasco JFM. Incidência de infecções bacterianas em pacientes queimados sob tratamento em hospital universitário de Curitiba. Rev Bras Queimaduras. 2011;10(1):3-9.
22. Martins CBG, Andrade SM. Queimaduras em crianças e adolescentes: análise da morbidade hospitalar e mortalidade. Acta Paul Enferm. 2007;20(4):464-9.
23. Raz-Pasteur A, Hussein K, Finkelstein R, Ullmann Y, Egozi D. Blood stream infections (BSI) in severe burn patients--early and late BSI: a 9-year study. Burns. 2013 Jun;39(4):636-42.
24. Zanetti G, Blanc DS, Federli I, Raffoul W, Petignat C, Maravic P, et al. Importation of Acinetobacter baumannii into a burn unit: a recurrent outbreak of infection associated with widespread environmental contamination. Infect Control Hosp Epidemiol. 2007;28(6):723-5.
25. Oliveira AC, Silva RS. Desafios do cuidar em saúde frente à resistência bacteriana: uma revisão. Rev Eletr Enferm [Internet]. 2008;10(1):189-97.
27. Pruitt Junior BA, McManus AT, Kim SH, Goodwin CW. Burn wound infections: current status. World J Surg. 1998 Feb;22(2):135-45.
26. Nasser S, Mabrouk A, Maher A. Colonization of burns wounds in Ain Shams University Burn Unit. Burns. 2003;29(3):229-33.
28. Macedo JL, Santos JB. Bacterial and fungal colonization of burn wounds. Mem Inst Oswaldo Cruz. 2005 Aug;100(5):535-9.
29. Hodle AE, Richter KP, Thompson RM. Infection control practices in U.S. burn units. J Burn Care Res. 2006 Mar/Apr;27(2):142-51.
30. Sengupta S, Kumar P, Ciraj AM, Shivananda PG. Acinetobacter baumannii: an emerging nosocomial pathogen in the burns unit Manipal, India. Burns. 2001 Mar;27(2):140-4.
31. Dean DA, Burchard KW. Fungal infection in surgical patients. Am J Surg. 1996 Mar;171(3):374-82.
1. Hospital Geral “José Pangella” de Vila Penteado, Departamento de Cirurgia Plástica
e Queimaduras, São Paulo, SP, Brazil.
Institution: Hospital Geral “José Pangella” de Vila Penteado, Departamento de Cirurgia Plástica e Queimaduras, São Paulo, SP, Brazil.
Analysis and/or data interpretation, conception and design study, conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, realization of operations and/or trials, resources, writing - original draft preparation, writing - review & editing.
Conception and design study, conceptualization, final manuscript approval, methodology, project administration, supervision, validation, visualization, writing - review & editing.
Corresponding author: Adriano Fernandes Araújo, Rua Juquis, nº391 - Apto 154B - Indianópolis, SP, Brazil, Zip Code: 040811-10. E-mail: adrianowb@hotmail.com
Article received: October 20, 2019.
Article accepted: February 22, 2020.
Conflicts of interest: none.








 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter