

Original Article - Year 2020 - Volume 35 -
Modified reduction mammoplasty for optimization of oncological approach: initial results and literature review
Mamoplastia redutora modificada para otimização de abordagem oncológica: resultados iniciais e revisão da literatura
ABSTRACT
Introduction: Breast cancer is the second most common cancer among women. Constant scientific evolution has allowed increasingly less invasive surgical approaches, reducing treatment-related morbidity without cancer damage. The objective of this article is to show the surgical results and the versatility of reduction mammoplasty with the modified Pitanguy technique to optimize the immediate reconstruction associated with cancer surgery.
Methods: We present the cases of three patients who underwent the proposed technique. Marking of the breast diagnosed with cancer is planned following the principles of reduction mammoplasty described by Pitanguy. However, the inferolateral resection triangle is transposed into the supratumoral area. It can be placed from the junction of the lateral quadrants (JLQ) to the superolateral quadrant (SLQ) of the oncological breast.
Results: the three patients underwent the described technique associated with contralateral symmetrization mammoplasty with the Pitanguy technique. All were submitted to adjuvant radiation therapy, associated or not with chemotherapy. Two patients progressed without incident and one had a small necrosis of 1x1 cm at the flaps lower junction in the breast with cancer, which was treated conservatively without delaying the adjuvant treatment. All were satisfied with the aesthetic result.
Conclusion: The described technique proved to be a good alternative for tumors located between the JLQ and the SLQ of the oncological breast. It provides broader resections and thus expands the indication for conservative surgery and reduces the need for radical surgery, with better aesthetic results without impairment of the cancer outcome
Keywords: Breast neoplasms; Mammoplasty; Mama; Segmental mastectomy; Breast diseases.
RESUMO
Introdução: O câncer de mama é a segunda neoplasia mais comum entre as mulheres. A constante evolução científica tem permitido abordagens cirúrgicas cada vez menos invasivas, diminuindo a morbidade relacionada ao tratamento sem prejuízo oncológico. O objetivo deste artigo é mostrar os resultados cirúrgicos e a versatilidade da mamoplastia redutora com a técnica de Pitanguy modificada, para otimização da reconstrução imediata associada à cirurgia oncológica.
Métodos: Apresentamos os casos de três pacientes submetidas à técnica proposta. A marcação da mama com diagnóstico de câncer é planejada seguindo os princípios da mamoplastia redutora descrita por Pitanguy. Porém, o triângulo de ressecção inferolateral é transposto para a área supratumoral. Ele pode ser posicionado da junção dos quadrantes laterais (JQL) até o quadrante superolateral (QSL) da mama oncológica.
Resultados: As três pacientes foram submetidas à técnica descrita associada à mamoplastia de simetrização contralateral com a técnica de Pitanguy. Todas realizaram radioterapia adjuvante, associada ou não à quimioterapia. Duas pacientes evoluíram sem intercorrências e uma apresentou pequena necrose de 1x1cm na junção inferior dos retalhos na mama com câncer, que foi tratada de maneira conservadora sem atrasar o tratamento adjuvante. Todas seguem satisfeitas com o resultado estético.
Conclusão: A técnica descrita mostrou-se uma boa alternativa para tumores localizados entre a JQL e o QSL da mama oncológica, proporcionando ressecções mais amplas e dessa forma ampliando a indicação de cirurgia conservadora e reduzindo a necessidade de cirurgia radical, com melhores resultados estéticos sem prejuízo do desfecho oncológico.
Palavras-chave: Neoplasias da mama; Mamoplastia; Mama; Mastectomia segmentar; Doenças mamárias
Introduction
Breast cancer is the most common cancer among women after skin tumors. According to data from the National Cancer Institute (INCA), the estimate of new cases in Brazil is 57,900 for 2018, which corresponds to 29% of all diagnosed neoplasms. Statistics indicate a progressive increase in its incidence, which represents 24.2% of the total number of female cancer cases in the world in 2018. It is the fifth leading cause of cancer death overall (626,679 deaths) and the most frequent cause of death from cancer in women1.
Malignant breast cancer has different histological types and diverse molecular profiles, allowing for increasingly individualized therapeutic approaches.
The constant scientific evolution, which generates a better understanding of breast cancer, has allowed surgical approaches that are less and less invasive, reducing treatment-related morbidity without cancer damage.
The first effective treatment for breast cancer was the radical mastectomy described by Halsted, in 18942, which was characterized by the resection of the breast (skin and gland), the two pectoral muscles and the three levels of the axillary lymph nodes in monoblock, obtaining low rates of local recurrence and good overall survival.
In 1948, Patey and Dyson3 published a series of cases of radical mastectomies with pectoralis major muscle preservation, obtaining results similar to classical radical surgery with less morbidity.
Later, in 1965, Madden4 published a study advocating the preservation of both pectoral muscles and obtained similar oncological results, with lower complication rates.
With their classic studies, begun in the 1970s, Veronesi et al., in 19815 and Fisher et al., in 19856, changed the paradigm of radicalism in breast cancer treatment. They noted that, in selected cases, breast-conserving (BCS) surgeries associated with radiation therapy provided oncological outcomes similar to those of radical surgeries and with a significant reduction in morbidity.
The main objective of BCS is the tumor resection with adequate margins, achieving favorable aesthetic results. Good aesthetic results are associated with a better quality of life for women with breast cancer7.
However, the main limitation of BCS is the relationship between the tumor resection area and the breast size. When this relationship is unfavorable, the observed surgical results are often unpleasant8.
Therefore, in this unfavorable scenario, the association between oncological resection technique and mammoplasty becomes an effective alternative to avoid radical surgery. This technical association allows breast resections of 20-40% without cosmetic damage9-11.
OBJECTIVE
In this article, we aim to show the surgical results and the versatility of the reduction mammoplasty recommended by Pitanguy, modified to optimize the immediate oncological approach and associated with the contralateral breast symmetrization.
METHODS
This is an observational, retrospective study, with a description of a series of consecutive clinical cases, carried out by reviewing medical records of patients operated and followed on an outpatient basis at the Brazilian Institute of Cancer Control (IBCC), São Paulo/SP.
It was approved by the Ethics Committee of the Research Institute and registered in the Brazil Platform (CAAE: 26068219.8.0000.0072). It was requested and accepted that patients’ Free and Informed Consent (ICF) should not be asked, as it is an observational and retrospective study.
Data were collected from 3 patients who underwent modified reduction mammoplasty for oncological optimization, associated with the contralateral breast symmetrization, during the period from August 2018 to July 2019.
Surgical planning
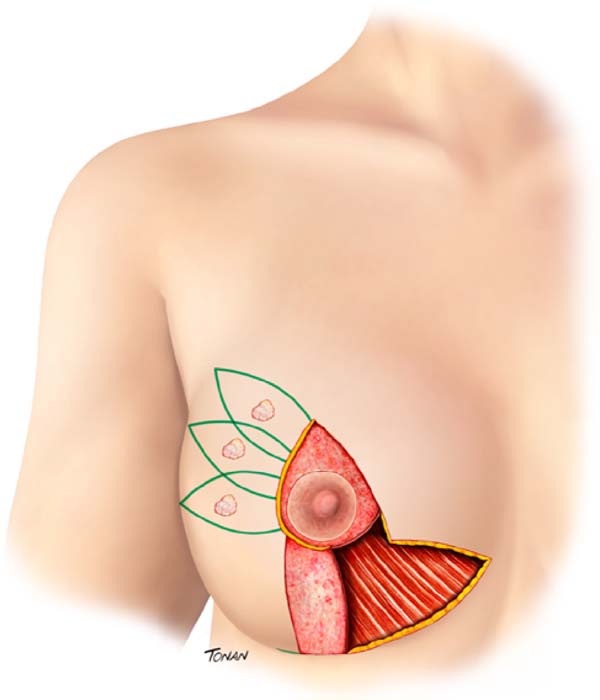
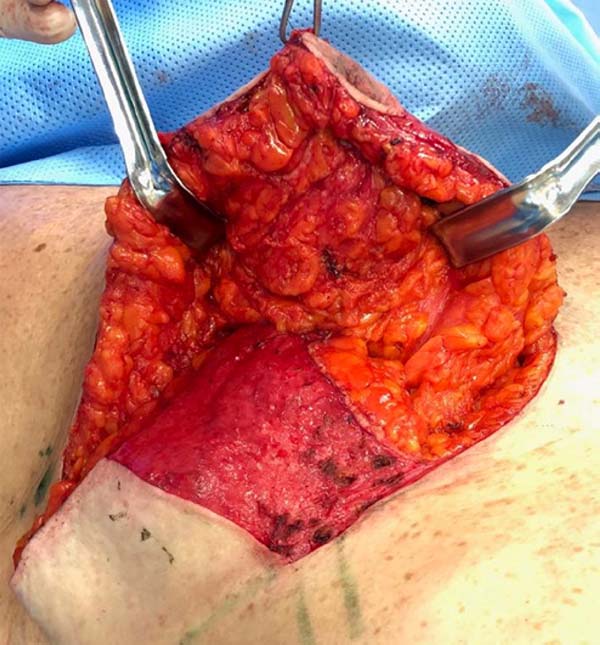
Surgical planning is performed with a multidisciplinary approach, that is, with the interaction between oncological resection performed by the mastology team and breast reconstruction with local tissues associated with contralateral breast symmetrization, performed by the plastic surgery team. Marking of the breast diagnosed with cancer is planned following the principles of reduction mammoplasty described by Pitanguy. However, the inferolateral resection triangle is transposed into the supratumoral area. The exact location of this transposed triangle is not fixed and is individually adapted for each patient, according to the location of the lesion, for the technique optimization. It can be placed from the junction of the lateral quadrants (JLQ) to the superolateral quadrant (SLQ) of the oncological breast (Figure 1).
The preferred pedicle to reposition the nipple-areola complex (NAC) is the superomedial one, since it does not interfere with oncological resection, even in lesions that reach the central region (CR) of the breast (Figure 1).
In lesions that achieve CR, wider resections are generally required to obtain satisfactory cancer margins. In these cases, to avoid an excessive reduction in breast volume, a de-epidermized flap of the lower breast region can be performed, with the objective of adequate volumetric replacement of the resected CR (Figure 1).
In lesions that reach the CR, broader resections are usually necessary to obtain satisfactory oncological margins. In these cases, to avoid excessive reduction in breast volume, a de-epidermized flap from the lower breast region can be performed, aiming at the adequate volumetric replacement of the resected CR (Figure 1).
The axillary approach, either to perform a sentinel node biopsy (SNB) or an axillary lymphadenectomy (AL), is performed through the same incision. Axillary access occurs through the transposed triangle end.
Symmetrization mammoplasty is performed following the principles of reduction mammoplasty described by Pitanguy, paying particular attention to leaving the oncological breast with a volume 10% greater than the contralateral breast, since adjuvant radiotherapy, mandatory in conservative surgeries, reduces approximately 10% of this breast volume.
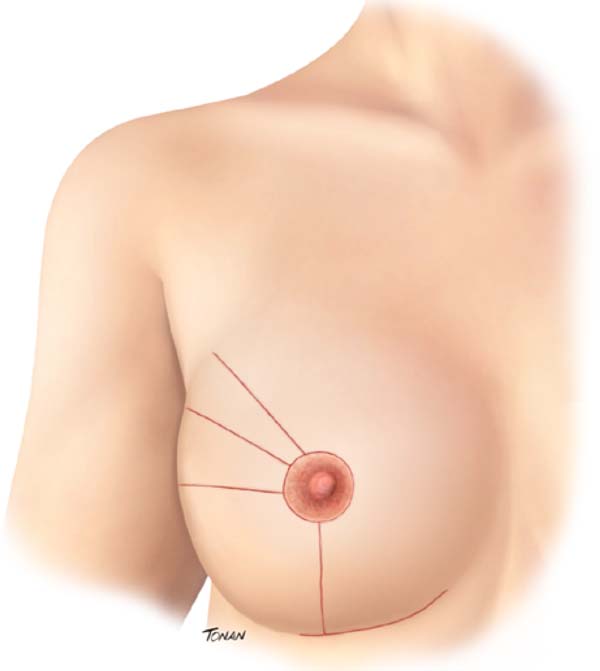
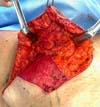
At the end of the procedure, the tumor bed is trimmed to help plan for adjuvant radiation therapy. Four radiopaque clips are applied to the cardinal points. The resulting scar in the oncological breast varies according to the supratumoral resection triangle position (Figure 2).
Results
The clinical, oncological and surgical data of the cases described are shown in Table 1.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age (years) | 44 | 53 | 64 |
| BMI (kg/m2) | 27.7 | 31.6 | 33 |
| Breast cancer surgery | ROLL | ROLL | 2 ROLL + 1 Needling |
| Axillary Surgery | SNB | SNB | SNB |
| Right breast weight (g) | 470 | 380 | 390 |
| Left breast weight (g) | 540 | 435 | 460 |
| Tumor size (mm) | 26 | 29 | 13 e 10 |
| Tumor Site | 2h ME | 11h MD | 9H MD |
| Histological type | IDC | IDC + IDCS | |
| Molecular subtype | Negative Triple | Luminal B HER2+ | Luminal B |
Case 1
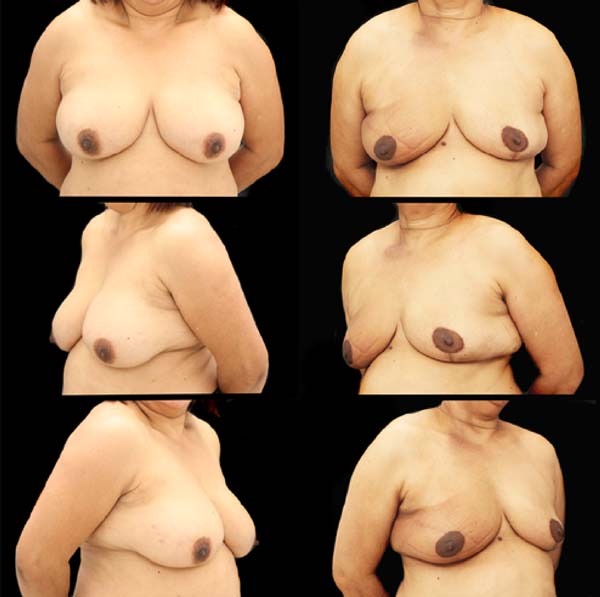
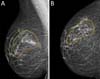
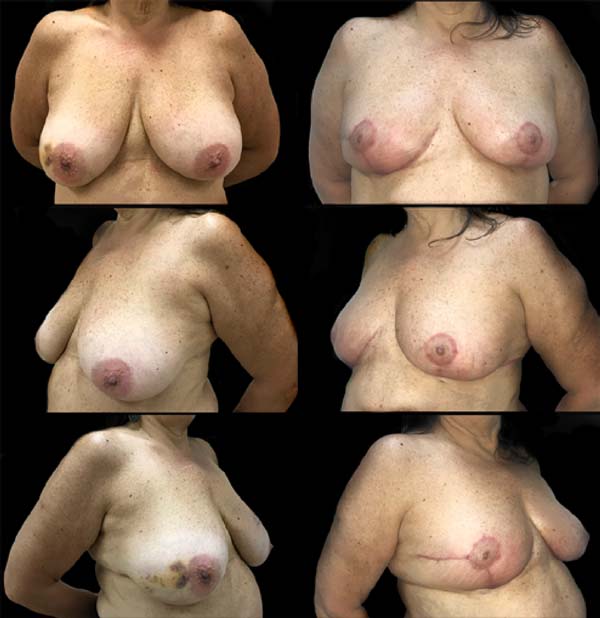
SDLF, 53 years old, diagnosed with invasive ductal carcinoma (IDC) of the right breast, pT2 pN1a, hybrid luminal subtype. Physical examination revealed breasts with grade 2 ptosis. The described technique was performed, with the transposed triangle placed at approximately 11 am on the right breast. The NACs were bilaterally repositioned with a superomedial pedicle. She underwent chemotherapy and adjuvant radiation therapy. The patient evolved without incident and was satisfied with the aesthetic result. Also, she was submitted to adjuvant radiation therapy. Photographic documentation with one year postoperative (Figure 3).
Case 2
CAGS, 44 years old, diagnosed with IDC of the left breast, triple-negative, cT2 cN0. Physical examination revealed breasts with grade 3 ptosis. She underwent neoadjuvant chemotherapy with carboplatin + paclitaxel, followed by doxorubicin + cyclophosphamide, which showed a complete pathologic response. Oncogenetic evaluation performed without evidence of pathological genetic mutations related to hereditary breast cancer. The described technique was performed, with the transposed triangle placed at approximately 2 am in the left breast. The NACs were bilaterally repositioned with a superomedial pedicle. Adjuvant radiotherapy was performed. The patient evolved without incident and was satisfied with the aesthetic result. Photographic documentation with one year postoperative (Figure 4).
Case 3
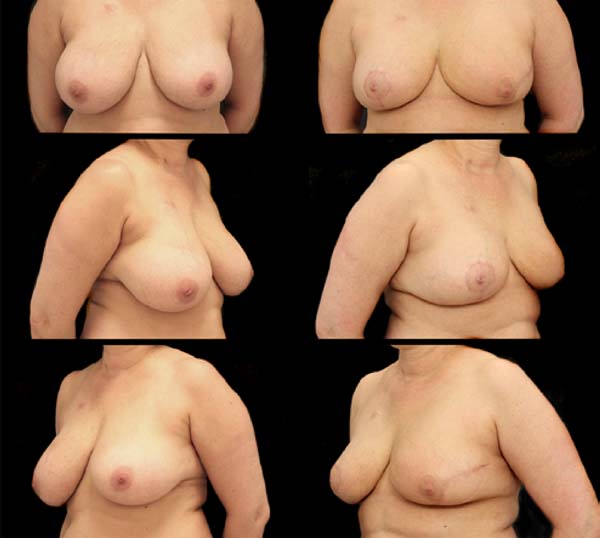
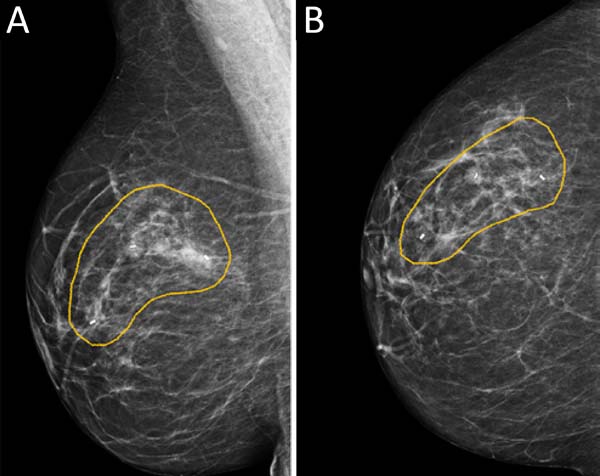
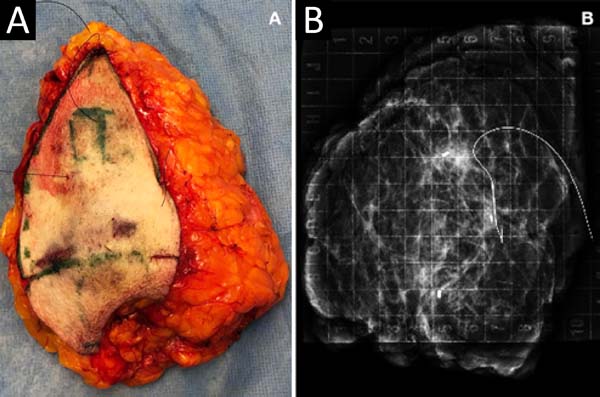
MCLPF, 64 years old, diagnosed with multicentric IDC, pT1 (m) pN0 (ls), luminal subtype B, associated with sclerosing intraductal papilloma in the right breast. Physical examination revealed breasts with grade 3 ptosis. Preoperative planning included 3 vacuum aspiration biopsy clips in the surgical resection area (Figure 5):
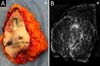
It was performed the described technique with the placement of the transposed triangle at approximately 9 a.m. on the right breast. In this case, due to the oncology need, surgical resection was extended to the CR, and the epidermis flap of the lower region was used for volumetric optimization of the reconstruction (Figures 6 and 7). The NACs were bilaterally repositioned with a superomedial pedicle.
The patient evolved with a small necrosis of 1x1 cm at the junction of the lower flap, in the right breast, which was treated conservatively, with good evolution. The patient evolved satisfied with the aesthetic result. Photographic documentation two months after surgery, before adjuvant radiotherapy (Figure 8).
Discussion
Classically, breast-conserving surgery (BCS) is indicated for women with unilateral breast cancer, up to 5 cm (T1 or T2) or tumors whose breast resection does not exceed 20 to 25% of the volume.
Conservative breast surgeries that require more extensive resections are associated with residual cosmetic deformities in up to 30% of cases12,13. In general, these changes have a significant impact on the quality of life of these women and result in enormous challenges for the correction or secondary aesthetic improvement of the breast due to technical difficulties and the risk of tissue mobilization in the irradiated breasts.
Thus, the oncological approaches associated with mammoplasty techniques, named as oncoplastic breast surgery (OBS), have as one of their main indications, the decrease in the rates of aesthetic dissatisfaction with conservative surgery associated with major breast resections14.
In addition to this vital indication, other practical applications of OBS are the technical possibility of performing breast-conserving surgery in multifocal, multicentric lesions and tumors larger than 5 cm (T3) or requiring breast resection greater than 25% of the breast volume, without aesthetic damage14. Classically, these cases would be indications for radical surgery.
In a recent literature review article, breasts showing aesthetically favorable surgical results were observed in 90.2% of patients who underwent OBS8. In women who underwent traditional BCS, the rates were 60 to 80%14,15.
Concerning oncological aspects, OBS presents results similar to BCS; that is, they present the same oncological safety. However, there is the advantage of providing lower repair rates due to inadequate surgical margins in OBS8.
Classically, the inverted “T” reduction mammoplasty technique, described in our country by Pitanguy, in 196716, is an excellent alternative for OBS when the oncological area to be resected is located in the area of the classic marking of the technique, generally at the lower breast pole. When this area is outside the mark, it is often necessary to make skin flaps, sometimes extensive, to access the desired location. This fact increases the risk of complications such as skin flaps necrosis, steatonecrosis, and dehiscence of the surgical wound.
Immediate repairs of tumors located in the superolateral quadrants of voluminous breast were addressed by Carramaschi et al., in 199117. The technique illustrated in this article was described by Ching et al., in 199718, it was presented in the II Latin American Convention of the European School of Oncology. In 201519,20, Silverstein et al., disseminated the technique on an international bibliographic base, calling it “ Oncoplastic Split Reduction.”
In the described technique, the inferolateral resection area was transposed to the breast superolateral region, more precisely from 9 to 11 a.m. in the right breast or from 1 to 3 a.m. in the left breast (Figure 1). We have to remember that approximately 50% of breast carcinomas occur in this location.
This modification of the reduction mammoplasty for the oncological approach allows a surgical technique with less breast devascularization, with easy access to the tumor region without the need to perform wide skin flaps. Besides, it has the possibility of supratumoral skin resection when it is oncologically indicated, which allows access to the axillary region without the need for an additional incision.
This technique results in surgical procedures with less risk of complications, a fact of extreme importance in cancer surgery, to reduce the risk of delay in the start of adjuvant treatment.
In the small series of cases presented, we observed that it is a safe, oncologically effective, versatile procedure for various tumor locations and with a high rate of patient satisfaction.
CONCLUSION
The described technique proved to be a good alternative for tumors located between the JLQ and the SLQ of the oncological breast, providing ample and safe resections.
ACKNOWLEDGMENT
To the medical illustrator Rodrigo Tonan, who masterfully created the figures used in the article.
REFERENCES
1. Instituto Nacional José de Alencar Gomes da Silva (INCA). Estimativa 2018: incidência do câncer de mama no Brasil [Internet]. Rio de Janeiro (RJ): INCA; 2018; [acesso em 2019 setembro 30]. Disponível em: http://www1.inca.gov.br/rbc/n_64/v01/pdf/15-resenha-estimativa-2018-incidencia-de-cancer-no-brasil.pdf
2. Halsted WS. I. the results of operations for the cure of cancer of the breast performed at the johns hopkins hospital from June, 1889, to January, 1894. Ann Surg. 1894 Nov;20(5):497-555.
3. Patey DH, Dyson WH. The prognosis of carcinoma of the breast in relation to the type of operation performed. Br J Cancer. 1948 Mar;2(1):7-13.
4. Madden JL. Modified radical mastectomy. Surg Gynecol Obstet. 1965 Dec;121(6):1221-30.
5. Veronesi U, Saccozzi R, Del Vecchio M, Banfi A, Clemente C, Delena M, et al. Comparing radical-mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981 Jul;305(1):6-11.
6. Fisher B, Bauer M, Margolese R, Poisson R, Pilch Y, Redmond C, et al. Five-year results of a randomized clinical-trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast-cancer. N Engl J Med. 1985 Mar;312(11):665-73.
7. Rowland JH, Desmond KA, Meyerowitz BE, Belin TR, Wyatt GE, Ganz PA. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. J Natl Cancer Inst. 2000 Sep;92(17):1422-9.
8. Papanikolaou IG, Dimitrakakis C, Zagouri F, Marinopoulos S, Giannos A, Zografos E, et al. Paving the way for changing perceptions in breast surgery: a systematic literature review focused on oncological and aesthetic outcomes of oncoplastic surgery for breast cancer. Breast Cancer. 2019 Jul;26(4):416-27.
9. Rainsbury RM. Training and skills for breast surgeons in the new millennium. ANZ J Surg. 2003 Jul;73(7):511-6.
10. Baildam AD. Oncoplastic surgery of the breast. Br J Surg. 2002;89(5):532-3.
11. Rew DA. Towards a scientific basis for oncoplastic breast surgery. Eur J Surg Oncol. 2003 Mar;29(2):105-6.
12. Curran D, van Dongen JP, Aaronson NK, Kiebert G, Fentiman IS, Mignolet F, et al. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC trial 10801. Eur J Cancer. 1998 Feb;34(3):307-14.
13. Kronowitz SJ, Feledy JA, Hunt KK, Kuerer HM, Youssef A, Koutz CA, et al. Determining the optimal approach to breast reconstruction after partial mastectomy - reply. Plast Reconstr Surg. 2006 Sep;118(3):813-4.
14. Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg. 2014 Feb;72(2):145-9.
15. Tenofsky PL, Dowell P, Topalovski T, Helmer SD. Surgical, oncologic, and cosmetic differences between oncoplastic and nononcoplastic breast conserving surgery in breast cancer patients. Am J Surg. 2014 Mar;207(3):398-402.
16. Pitanguy I. Surgical treatment of breast hypertrophy. Br J Plast Surg. 1967 Jan;20(1):78-85.
17. Carramaschi F, Yamaguchi C, Herson M, Alonso N, et al. Immediate breast reconstruction after quadrantectomy. Rev Soc Bras Cir Plast. 1991;6(3):73-7.
18. Ching AW, Tietnens M, Garcia EB, Huffembaucher R, Carramaschi F, et al. Breast reconstruction in the upper-external quadrantectomy associated eith eight reduction mammaplasty. In: II Convenção Latino-Americana do European School of Oncology - ESO. São Paulo, Brasil. São Paulo (SP): Comitê Brasileiro da ESO; 1997. p. 22.
19. Silverstein MJ, Savalia N, Khan S, Ryan J. Extreme oncoplasty: breast conservation for patients who need mastectomy. Breast J. 2015 Jan/Feb;21(1):52-9.
20. Silverstein MJ, Savalia NB, Khan S, Ryan J, Epstein M, DeLeon C, et al. Oncoplastic split reduction with intraoperative radiation therapy. Ann Surg Oncol. 2015;22(10):3405.
1. Instituto Brasileiro de Controle do Câncer (IBCC), Serviço de Cirurgia Plástica,
São Paulo, SP, Brasil.
Corresponding author: Gabriel Salum D’Alessandro, Av. Alcântara Machado, nº 2576 - Mooca, São Paulo, SP, Brazil. Zip Code 03102-002 E-mail: gsdalessandro@yahoo.com.br
Article received: December 22, 2019.
Article accepted: March 02, 2020.
Conflicts of interest: none.





 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter