

Case Report - Year 2020 - Volume 35 -
Pyoderma gangrenosum after trauma to the dorsum of the hand
Pioderma gangrenoso em dorso de mão pós-trauma
ABSTRACT
Introduction: Cullen’s postoperative gangrene, also called pyoderma gangrenosum (PG) or sterile neutrophilic abscess, was first described in the medical literature by Cullen in 1924. Later Brusting et al., in 1930, described PG in more detail.
Objective: To report a rare case of pyoderma gangrenosum (PG) in an extraneous limb that was triggered by blunt trauma to the dorsum of the hand.
Discussion: A histopathological exam is not sufficient to diagnose PG, Therefore, a PG diagnosis is based on clinical evidence. The clinical presentation is variable and includes rare bullous, pustular and vegetative forms. Other rare forms of PG occur at sites of pathergy (20-30%), periostomal skin, the dorsum of the hand, the head and neck. PG can also be multisystemic and paraneoplastic.
Conclusion: The appearance of sudden-onset PG is rare in the dorsum of the hand. It is important to classify PG’s clinical forms, and to establish associations between PG and underlying pathologies. It is also very important to avoid surgery during early PG. The many drugs used to treat PG, and the multiple patient responses, demonstrate the difficulty of standardizing treatment. Physicians may have to use an empirical approach to select the appropriate drug for each patient.
Keywords: Pyoderma gangrenosum; Wounds and injuries; Multiple trauma; Upper extremity; autoimmune diseases.
RESUMO
Introdução: A gangrena pós-operatória de Cullen, também denominada de pioderma gangrenoso (PG) ou abscesso neutrofílico estéril, pelo fato das lesões cutâneas não conterem micro-organismos patogênicos teve sua primeira aparição na literatura médica no ano de 1924, pelo relato feito por Cullen, segundo Schofer e Baur. Mais tarde Brusting et al., em 1930, acrescentaram maiores detalhes a descrição inicial.
Objetivo: Relatar um caso raro de pioderma gangrenoso (PG) em extremidades de membros desencadeado por um trauma contuso em dorso de mão.
Discussão: O exame histopatológico do PG não é diagnóstico, portanto, a elucidação do quadro se baseia em evidências clínicas. A apresentação clínica é variável: a bolhosa, pustulosa e vegetante. Outras formas raras incluem o PG em locais de patergia (20-30%), periostomal, dorso da mão, PG maligno ou da cabeça e pescoço, multisistêmico e paraneoplásico.
Conclusão: O aparecimento do PG é de início súbito e raro em dorso de mão. É importante classificar sua forma clínica, estabelecer associações com patologias de base. A diversidade de drogas para a terapêutica demonstra a dificuldade de padronização de tratamento com variadas respostas, que pode ser empírico e evitar abordagem cirúrgica precoce.
Palavras-chave: Pioderma gangrenoso; Ferimentos e lesões; Traumatismo múltiplo; Extremidade superior; Doenças
INTRODUCTION
Postoperative Cullen gangrene, also called pyoderma gangrenosum (PG)1 or sterile neutrophilic abscess (because lesions do not contain pathogenic micro-organisms) was first described in the medical literature by Cullen in 19241. Brusting et al. then described PG in greater detail in 19302.
The etiology of PG is unknown. In most cases, PG is related to malignant neoplasms, rheumatologic diseases, arthritis, inflammatory bowel diseases such as ulcerative colitis or Crohn’s disease, monoclonal gammopathies, collagenosis, Behcet’s disease, Wegener’s granulomatosis, myeloproliferative diseases, and infectious diseases, especially hepatitis and Acquired Immunodeficiency Syndrome (AIDS)2.
PG is characterized by painful ulcers of various sizes and depths with ill-defined borders. PG has no neoplastic origin, nor is it associated with primary vasculitis. Though PG lesions may suffer from secondary infection, the underlying pathology of PG is unrelated to infection3.
Pyoderma gangrenosum is a rare inflammatory neutrophilic dermatosis occurring in 3 to 10 cases per million per year. In most cases, PG is chronic and relapsing. The condition commonly affects adults between 20 and 50 years old, and is more common in women than men. Children and adolescents constitute only 4% of cases1-5. One retrospective study found that the incidence of PG in Brazil was 0.38 cases per 10,000 hospital visits6.
In this report we detail a rare case of pyoderma gangrenosum caused by blunt trauma to the dorsum of the hand. The patient was treated in the Plastic Surgery Unit of t he Regional Hospital of Asa Norte (HRAN), Brasília-DF.
CASE REPORT
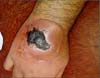
A 33-year-old male Caucasian patient was the victim of a work-related accident with a sledgehammer, resulting in trauma to the dorsum of the right hand (Figure 1). The patient was a mechanic and did not report having any comorbid conditions.
Edema appeared immediately after the trauma, which developed into a large hematoma over the next few days. The hematoma was surgically drained at the patient’s local health clinic. After the drainage, a chronic wound formed on the dorsum of the patient’s hand (Figure 1).
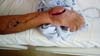
The patient was referred to a specialist in Goiânia, and was subjected to a Chinese flap procedure (Figure 2). Ten days after the surgery, the edge of the flap detached from the wound bed. Necrosis at the wound edges soon spread, resulting in the total loss of the flap (Figure 2). After debridement and wound preparation, a biopsy of the exudative found signs of chronic inflammation and negative cultures.
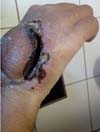
A new flap procedure was performed 36 days after the loss of the first. The new posterior interosseous fasciocutaneous flap underwent the same evolution as the first. The edge of the flap detached from the wound bed, after which necrosis spread outwards from the wound’s edges. Although without any early suffering in the two vascular procedures, a new wound reopened (Figures 3 and 4).
The patient was then referred to our Plastic Surgery Unit at the HRAN in Brasilia. A superficial debridement of the necrotic tissue was performed, and new tissue and bone samples were collected for culture and biopsy. The resulting histopathological tests were inconclusive. An AFB test was negative, microscopy for leishmania was negative, and cultures for fungus, tuberculosis, and aerobic bacteria were negative. The patient only displayed signs of acute chronic inflammation without signs of neoplasia.
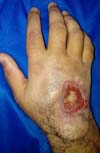
After clinical discussion within the unit and exclusion of other diagnoses, we suspected pyoderma gangrenosum. No further surgical debridement was performed in the HRAN. The patient began systemic corticosteroid therapy (maximum dose of prednisone: 70 mg 1x/day) and triamcinolone was applied to the edge of the wound. The patient’s condition improved, and the wound almost spontaneously closed after showing signs of granulation (Figure 5). A few months after the patient was discharged, he once again experienced necrosis resulting in joint pain and bone exposure. Administration of morphine in the patient’s home city resulted in only partial analgesia.
The patient was treated by his local rheumatologist and orthopedist, who treated him with corticosteroid therapy. The patient then suffered from a bone infection. Complementary radiological exams were performed. The patient showed signs of acute osteomyelitis with onset of sepsis, and reported that his joint pain did not respond to intravenous morphine. Physicians therefore opted to amputate the patient’s hand at the wrist (Figure 6).
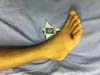
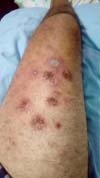
The patient symptoms significantly improved after surgery, and the patient did not experience further pain. However, the patient showed signs of pathergy in his lower limbs after a mild local trauma to his ankle. The trauma triggered the opening of new wounds in the dorsum of the foot and in the anterior portion of the thigh. The patient was treated with the immunomodulator Adalimumab along with Methotrexate, after which the patient’s wounds improved (Figures 7 and 8).
DISCUSSION
Because a histopathological exam is not sufficient evidence to diagnose PG, a PG diagnosis is based on clinical evidence. Edema, signs of massive inflammation caused by neutrophils, engorgement, thrombosis of small and medium vessels, necrosis, and hemorrhage are often seen in PG patients. The formation of abscesses and necrosis results in tissue liquefaction and secondary thrombosis of venules, allowing PMN leukocytes to infiltrate the region. The lesions originate from suppurative granulomatous dermatitis, and regress with remarkable fibroplasia6.
There is evidence to suggest that PG is an autoimmune disease, specifically caused by immune-induced cellular autophagy. This explanation is consistent with this case, and would help explain the loss of the two flaps constructed to cover the patient’s initial wound6,7.
The immunological reaction involves capillary vasodilation, causing a cutaneous rash. This process initiates a sequence of reactions, resulting in intense neutrophil migration to the region. The neutrophils remain in the basal layer of the skin and produce large amounts of collagenase, which degrades both the collagen and the capillaries of the basal layer. The bonds holding the basal layer together then degrade, opening the skin and causing necrosis. Necrosis, in turn, attracts even more neutrophils and macrophages to the site. These neutrophils produce more collagenase that destroys more cutaneous tissue, resulting in a positive feedback cycle6,7.
The clinical presentations of PG are variable, and include the rare bullous, pustular, and vegetative forms of PG. Even more uncommon forms include PG at sites of pathergy (in 20-30% of cases), the periostomal skin, the dorsum of the hand, and the head and neck. PG can also be multisystemic and paraneoplastic6,7.
PG usually begins as a deep and painful nodule or a superficial hemorrhagic pustule, sometimes resulting from minor trauma. A painful and ulcerated lesion with irregular borders then forms. These lesions have an inflammatory and elevated aspect, appear dark reddish or purple, and have a granular necrotic background with slight abscesses6,7.
In their ulcerated form, the interiors of PG lesions produce a hemorrhagic, purulent exudate. These lesions spread in a serpiginous pattern through the excavation of lesion edges or the appearance of new hemorrhagic pustules.
When superficial, PG lesions can be confined to the dermis. However, more commonly PG lesions expand into subcutaneous tissue and fascia, ultimately exposing muscle and bone.
Multiple PG lesions can appear gradually and simultaneously in different locations, especially in the lower limbs, abdomen and buttocks. Interestingly, the mucous membranes are usually spared. However, aphthous lesions may occur in the oral cavity, sometimes massively involving the pharynx and larynx.
PG can progress in two main patterns: (1) an explosive beginning involving the rapid spread of lesions, pain, fever, systemic toxicity, hemorrhagic phlyctenae, suppurations, and an inflamed halo around wound edges; or (2) a slow progression involving massive granulations inside ulcers, crust and
hyperkeratosis at wound edges, and the spontaneous regression of some lesions while other lesions progress eventually spreading to extensive areas8,9.
The four distinct clinical and histopathological forms of PG are:
Propagation - corresponding to 12.5% of cases10: Also called superficial granulomatous pyoderma, this is the most localized and least aggressive form of PG. Patients present with
superficial lesions that have verrucous aspects and non-purulent backgrounds. Lesions
occur on the torso, head and neck. Rapidly responds to appropriate therapy.
Bullous - 6.25% of cases10: lesions have an acute onset and are associated with leukemic frameworks. Lesions
are superficial and involve papules, purpura and bluish bullae, and hemorrhaging.
Ulcerative - 81.52% of cases10: lesions begin as small pustules surrounded by an inflammatory halo. Lesions are
painful and evolve rapidly. As lesions resolve, an atrophic scar and an epidermis
with a “cigarette paper-like” aspect are visible11.
Pustular - a rare presentation associated with fever, arthralgia, and intestinal
inflammatory diseases. Lesions occur mainly in the extensor surface of the extremities.
After the intestinal pathology is controlled, the disease may regress without leaving
scars, although the lesions may coexist with the ulcerative form10,11.
PG is diagnosed clinically, mostly by excluding similar presentations. Cultures for fungi and bacteria are generally negative, and the results of histopathological examination are compatible with neutrophilic dermatosis6-11. These general clinical features were consistent with this case, and the results of multiple biopsies and negative cultures are consistent with a diagnosis of ulcerative and pustular PG (Figure 8).
PG may appear after trauma or surgery, and is often confused with the infection of a surgical wound. Proper treatment for PG is performed systemically, and importantly surgical debridement is not recommended due to its potential to promote pathergy12,13.
To illustrate this principle, the two surgical procedures performed in this case resulted in flap loss, and local debridement reactivated and worsened the patient’s necrosis.
In 50 to 70% of patients, PG is associated with an underlying disease such as inflammatory bowel disease, rheumatic diseases, hematological diseases or malignancy, hepatitis B and C, AIDS, systemic lupus erythematosus, psoriasis, or reactive arthropathies3,14,15.
In this case, there was no other pathology associated with PG.
Fever, malaise and myalgias have been reported in some PG patients. Variable presentations of arthritis are present in 37% of cases, which include classic arthritis, asymmetric arthritis in the lower limbs, and monoarthritis3. In this case, the patient presented with high intensity arthralgia and fever, and myalgia that was unresponsive to morphine.
Pathergy can occur in up to 25% of cases, in which new lesions appear as a result of trauma such as insect bites, intravenous injections, debridement, and even biopsy. Pathergy can also occur after gynecological surgical procedures as reported by Meyer et al. in 200616, after partial thickness skin grafts as reported by Coltro et al. in 200617, and after mammoplasty as reported by Soares et al. in 201318.
Pathergy was also observed in this case. Specifically, the patient’s condition deteriorated after surgical trauma and debridement. Pathergy was also observed after mild trauma to the foot, which led the emergence of a new lesion next to the trauma site (Figures 7 and 8).
The bullous form of PG is also associated with hematological disorders such as leukemia and myelodysplastic syndrome (MDS). Indeed, 54% of bullous PG cases are associated with leukemia18.
Therapeutic alternatives include immunomodulators, corticosteroids, and immunosuppressants. The patient’s treatment depends on the specific presentation of PG, as well as the doctor’s experience regarding proper drug selection19.
PG is treated through immunosuppression. Local treatments should only be used for very small and early lesions. The affected area should be kept clean and moist. Occlusive dressings or hydrogel can be used.
Surgical debridement should be avoided, since the resulting pathergy can worsen the patient’s condition.
Intralesional triamcinolone infiltration (20 mg/ mL in monthly applications) can lead to remission after five to eight weeks3. While such treatment improved our patient’s condition initially, his condition worsened near the end of triamcinolone treatment (Figure 5).
Biweekly intralesional injections of cyclosporine (1:3 solution in saline) are another alternative treatment for PG.
The vast majority of PG patients undergo systemic treatment, while local therapy is used as adjuvant. High doses of corticosteroids (prednisone or prednisolone orally, 1-3 mg/kg/day) can be administered during the early stages of PG. Corticosteroids can also be administered in the form of pulse therapy (methylprednisolone 1 g/day for three days). After the disease has been controlled, the dose of corticosteroids is gradually reduced. However, in this case the patient did not respond to corticosteroid therapy20-23.
Cyclosporine is the gold standard treatment for PG. The majority of PG cases respond well to relatively low doses of cyclosporine (3-6 mg/kg/day). It is important to monitor blood pressure, renal function, hepatic function, and triglyceride levels during cyclosporine therapy. Other options include tacrolimus, azathioprine, dapsone, thalidomide, and clofazimine21.
Recent reports show that Infliximab can successfully treat cases of PG that were resistant to other therapies21. A combination of methotrexate and adalimumab produced a good clinical response in our patient.
Because the fundamental therapeutic principle PG treatment is immunosuppression, the possibility of infection must be completely excluded before commencing treatment.
Surgery has therapeutic value in the final stages of PG. After total remission of PG, grafts or flaps may be required to close large areas affected by skin loss23.
Pyoderma gangrenosum of the hand is rare and is usually confused with infection. In a series of seven cases published by Huish et al. in 200124, PG was misdiagnosed 13 times (ranging from 1-3 times per patient) resulting in 16 unnecessary surgeries (an average of 2.2 per patient) including four amputations and two failed skin grafts. Incorrect diagnoses lead to unnecessary treatments, surgeries, and even the appearance of sudden-onset PG 24.
It is important to classify PG’s clinical forms, establish associations between PG and underlying pathologies, and to evaluate the immune response in PG. The many drugs used to treat PG demonstrate the difficulty of standardizing treatment. Physicians can use an empirical approach to select the appropriate drug for each patient. The administration of corticosteroids, immunosuppressants and immunomodulators (combined with local care) can stop the progression of the disease. There are also rare reports of resistance to these medications. Such patients have a reserved prognosis, as in our case, in which worsening PG eventually led to amputation.
REFERENCES
1. Schofer H, Baur S. Successful treatment of postoperative pyoderma gangrenosum with cyclosporin. J Eur Acad Dermatol Venereol. 2002;16(2):148-51.
2. Brusting LA, Goeckerman WH, O’Leary PA. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in 5 cases occurring in adults. Arch Derm Syphilol. 1930;22(4):655-80.
3. Blitz NM, Rudikoff D. Pyoderma gangrenosum. Mt Sinai J Med. 2001;68(4-5):287-97.
4. Serra-Baldrich E, Boixareu MJT. Pioderma gangrenoso: consideraciones. Act Dermatol. 2001;4(2):115-22.
5. Tanus R, Cassol T, D’Aquino Neto V. Pioderma gangrenoso em membro inferior: relato de caso. Arq Catarin Med. 2009;38(Supl 1):70-2.
6. Graças AM, Alecrim ES, Lyon S. Pioderma gangrenoso: evidências clínicas e características. Rev Med Minas Gerais. 2016;26:e-1790.
7. Konopka CL, Padulla GA, Ortiz MP, Beck AK, Bitencourt MR, Dalcin DC. Pioderma gangrenoso: um artigo de revisão. J Vascul Bras. 2013;12(1):25-33.
8. Hadi A, Lebwohl M. Clinical features of pyoderma gangrenosum and current diagnostic trends. J Am Acad Dermatol. 2011;64(5):950-4.
9. Wani I, Bhat IHG, Mir M, Mir M, Hassan N, Mustafa A. Pyoderma gangrenosum of abdominal wall: a case report. Oman Med J. 2011;26(1):64-5. DOI: http://doi.org/10.5001/omj.2011.18
10. Conrad C, Trüeb RM. Pyoderma gangrenosum. J Dtsch Dermatol Ges. 2005;3(5):334-42. DOI: http://dx.doi.org/10.1111/j.1610-0387.2005.05022.x
11. Newman B, Cescon D, Domenchini A, Siminovitch KA. CD2BP1 and CARD 15 mutations are not associated with pyoderma gangrenosum in patients with inflammatory bowel disease. J Invest Dermatol. 2004;122(4):1054-5. DOI: http://dx.doi.org/10.1111/j.0022-202X.2004.22430.x
12. Zold E, Nagy A, Devenyi K, Zeher M, Barta Z. Successful use of adalimumab for treating fistulizing Crohn’s disease with pyoderma gangrenosum: two birds with one stone. World J Gastroenterol. 2009;15(18):2293-5. DOI: http://dx.doi.org/10.3748/wjg.15.2293
13. Souza CS, Chioss MPV, Takada MH, Foss NT, Roselino AMF. Pioderma gangrenoso: casuística e revisão de aspectos clínico-laboratoriais e terapêuticos. An Bras Dermatol. 1999;74(5):465-72.
14. Beber AAC, Knob CF, Shons KRR, Neumaier W, Silva JCN, Monticielo OA. Pioderma gangrenoso associado à artrite reumatoide: descrição de caso. Rev Bras Reumatol. 2014;54(4):322-5.
15. Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165(6):1244-50. DOI: https://doi.org/10.1111/j.1365-2133.2011.10565.x
16. Meyer TN. Pioderma gangrenoso: grave e mal conhecida complicação da cicatrização. Rev Bras Cir Plást. 2006;21(2):120-4.
17. Coltro PS, Valler CS, Almeida PCC, Gomez DS, Ferreira MC. O papel da patergia no pioderma gangrenoso em áreas doadoras de enxertos cutâneos: relato de caso. Rev Bras Cir Plást. 2006;21(4):231-5.
18. Soares JM, Rinald AE. Pioderma gangrenoso pós-mamoplastia redutora: relato de caso e discussão. Rev Bras Cir Plást. 2013;28(3):511-4.
19. Batista MD, Fernandes RL, Rocha MAD, Ikino JK, Pinheiro RF, Chauffaille MLF, et al. Pioderma gangrenoso bolhoso e síndrome mielodisplásica. An Bras Dermatol. 2006;81(5 Supl 3):S309-12.
20. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211. DOI: http://dx.doi.org/10.2165/11595240-000000000-00000
21. Lazarus GS, Goldsmith LA, Rocklin RE, Pinals RS, De Buisseret JP, David JR, et al. Pyoderma gangrenosum, altered delayed hypersensitivity and polyarthritis. Arch Dermatol. 1972;105(1):46-51. DOI: http://dx.doi.org/10.1001/archderm.1972.01620040018003
22. Friedman S, Marion JF, Scherl E, Rubin PH, Present DH. Intravenous cyclosporine in refractory pyoderma gangrenosum complicating inflammatory bowel disease. Inflamm Bowel Dis. 2001;7(1):1-7. DOI: http://dx.doi.org/10.1097/00054725-200102000-00001
23. Miller J, Yentzer BA, Clark A, Jorizzo JL, Feldman SR. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62(4):646-54. DOI: http://dx.doi/10.1016/j.jaad.2009.05.030
24. Huish SB, Bountiful UT, De La Paz EM, Cincinnati OH, Ellis PRIII, Dallas TX, et al. Pyoderma gangrenosum of the hand: a case series and review of the literature. J Hand Surg Am. 2001;26(4):679-85.
1. Hospital Regional da Asa Norte, Brasília, DF, Brazil.
Corresponding author: Altino Vieira de Rezende Filho Neto, Setor SMAS, Trecho 1, Lote C, Bloco J, Brasília, DF, Brazil. Zip Code: 71218-010. E-mail: altinofn@hotmail.com
Article received: January 21, 2019.
Article accepted: April 21, 2019.
Conflicts of interest: none.













 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter