

Case Report - Year 2020 - Volume 35 -
Breast implant-associated anaplastic large cell lymphoma: a diagnostic challenge
Linfoma anaplásico de células grandes associado a implante mamários: um desafio diagnóstico
ABSTRACT
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a provisional entity with morphological and immunophenotypic characteristics indistinguishable from ALK-negative anaplastic large cell lymphoma (ALCL). Unlike ALCL, BIA-ALCL arises mainly in association with breast implantation. Diagnostic confirmation of BIA-ALCL can be difficult and associating morphological and pathological hallmarks with flow cytometry and immunohistochemistry can assist in the diagnosis. The objective of this report is to describe a case of BIA-ALCL in which cytological and immunophenotypological analysis using flow cytometry suggested the presence of large CD30-positive cells in the effusion fluid.
Keywords: Anaplastic large cell lymphoma, Flow cytometry, Breast implant, Cytology, CD30 ligand.
RESUMO
O linfoma anaplásico de grandes células associado a implantes mamários (BI-ALCL) é uma entidade provisória com características morfológicas e imunofenotípicas indistinguíveis do linfoma anaplásico de grandes células ALK-negativo (ALCL), porém surge principalmente em associação com um implante mamário. A confirmação do diagnóstico pode ser difícil, e a associação da presença das células típicas na morfologia e anatomia patológica com exames de citometria de fluxo e imunohistoquímica pode auxiliar no diagnóstico. O objetivo deste estudo é descrever um caso de BI-ALCL em que a citologia e a análise da imunofenotipagem por citometria de fluxo sugeriram a presença de células grandes positivas para CD30 no fluido de efusão.
Palavras-chave: Linfoma anaplásico de células grandes, citometria de fluxo, implante mamário, citologia, ligante CD30
INTRODUCTION
The 2016 World Health Organization’s (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues1 recognizes Breast Implant- Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) as a provisional entity, with morphological and immunophenotypic features indistinguishable from those of ALK-negative anaplastic large cell lymphoma (ALCL). Unlike ALCL, BIA-ALCL arises primarily in association with breast implantation2.
BIA-ALCL is a very rare disease (1 case per 1-3 million women with implants), which may be localized to the seroma cavity, or may involve the pericapsular fibrous tissue. Most patients present with a peri-implant effusion, and present less frequently with a mass. Diagnosis is performed by aspirating the effusion around the implant and confirming the CD30-positivity of cells within the sample. However, confirming the diagnosis may be difficult. Associating of presence of hallmark cells with the results of flow cytometry and immunohistochemistry can aid accurate diagnosis3-4.
Most patients have an excellent prognosis upon complete removal of the capsule, and upon surgically implanting a prosthesis with negative margins.5-6.
OBJECTIVE
To describe a BIA-ALCL case in which cytology and flow cytometry analysis suggested the presence of CD30-positive large cells in the effusion fluid.
CASE REPORT
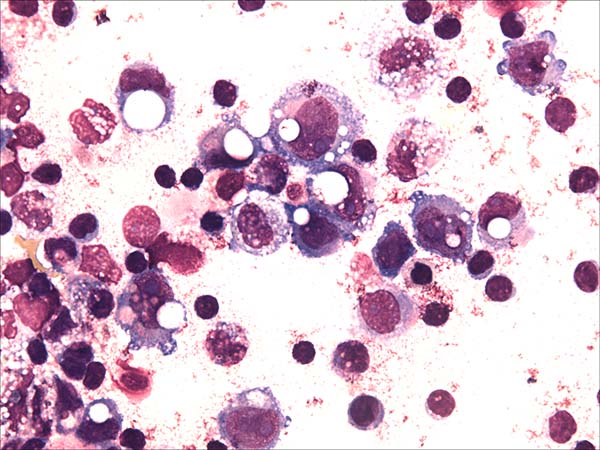
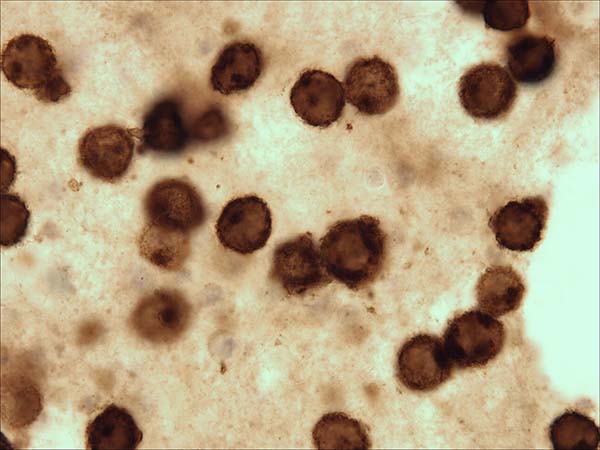
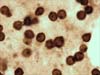
A 52-year-old woman with a history of breast cancer presented with left breast swelling and local pain. Seven years before, she had undergone a modified radical mastectomy of her left breast and had thereafter undergone immediate breast reconstruction with tissue expander. She had then developed a surgical infection, and shortly thereafter had the expander removed. Six months after completing radiotherapy, she had undergone another breast reconstruction using a latissimus dorsi flap and textured anatomical-shaped implant. Upon presentation, imaging revealed a peri-implant effusion. Approximately 100 ml of cloudy, yellow fluid was collected and immediately sent to the flow cytometry lab. Cytological examination revealed numerous large, anaplastic cells with pleomorphic nuclei, prominent nucleoli, and moderate basophilic cytoplasm with frequent vacuoles (Figure 1). Multiparametric flow cytometry (MFC) immunophenotyping revealed large tumor cells (increased FSC/SSC scatter) with bright expression of CD30, CD45, CD25 and HLA-DR, as well as the absence of CD3 expression within T-lineage cells and a lack of the B-cell antigens CD19 and CD20 (Figure 2). The patient underwent a bilateral breast implant removal and a total capsulectomy. Pathological examination of the seroma then confirmed the presence of clustered large lymphoma cells that were immunohistochemically positive for CD30 and negative for CD20 and CD3 (Figure 3). However, histologic sections of the breast capsule showed only fibrin admixed with infiltrating reactive lymph histiocytes.
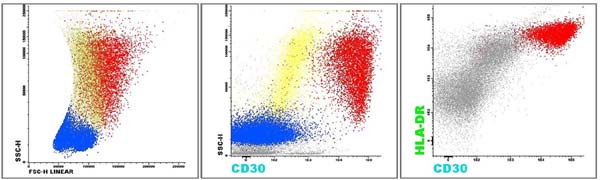
MFC was performed using an 8-color Becton Dickinson FACS Canto II cytometry system with FACS Diva 8 software for data acquisition, and Infinicyt™ for flow cytometry analysis. The neoplastic cell population exhibited bright co-expression of CD30, CD25 and HLA-DR, which was confirmed by immunohistochemistry of the seroma fluid. While this bright expression pattern may not be specific for ALCL, it is easily identifiable and may thus increase the sensitivity of BI-ALCL detection.
It is important to emphasize that the flow cytometry sample was immediately sent to the laboratory, in natura and at room temperature, and was immediately processed to prevent cell destruction and the loss of antigen strength.
CONCLUSION
CD30-positive BI-ALCL is a rare type of T-cell lymphoma that remains a diagnostic challenge. The challenging nature of BI-ALCL diagnosis underscores the importance of correlating precise immunophenotypic analysis with morphologic evaluation and clinical pathology. Multiparameter flow cytometry can aid in the diagnostic evaluation of effusions or tissue samples in association with breast implantation/prostheses.
COLLABORATIONS
|
APDA |
Analysis and/or data interpretation, Conception and design study, Conceptualization, Data Curation, Final manuscript approval, Realization of operations and/or trials, Resources, Writing - Original Draft Preparation, Writing - Review & Editing |
|
AG |
Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Writing - Review & Editing |
|
JJ |
Analysis and/or data interpretation, Data Curation, Writing - Review & Editing |
|
FG |
Analysis and/or data interpretation, Data Curation, Final manuscript approval |
|
SKN |
Analysis and/or data interpretation, Data Curation, Final manuscript approval, Resources |
REFERENCES
1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. World Health Organization (WHO) - Classification of tumors of haematopoietic and lymphoid tissues. Geneva: WHO; 2016. v. 2.
2. Taylor CR, Siddiqi IN, Brody GS. Anaplastic large cell lymphoma occurring in association with breast implants: review of pathologic and immunohistochemical features in 103 cases. Appl Immunohistochem Mol Morphol. 2013;21(1):13-20.
3. Wu D, Allen C, Fromm JR. Flow cytometry of ALK-negative anaplastic large cell lymphoma of breast implant-associated effusion and capsular tissue. Cytometry Part B Clin Cytom 2015;88(1):58-63.
4. Montgomery-Goecker C, Fuda F, Krueger JE, Chen W. Immunophenotypic characteristics of breast implant-associated anaplastic large-cell lymphoma by flow cytometry. Cytometry Part Clin Cytom. 2015;88(5):291-3.
5. Miranda RN, Aladily TN, Prince HM, Kanagal-Sharmanna R, Jong D, Fayad LE, et al. Breast implant-associated anaplastic large cell lymphoma: long term follow-up of 60 patients. J Clin Oncol. 2014;32(2):114-20.
6. Kaartinen I, Sunela K, Alanko J, Hukkinen K, Karjalainen-Lindsberg ML, Svarvar C. Breast implant-associated anaplastic large cell lymphoma - From diagnosis to treatment. Eur J Surg Oncol. 2017;43(8):1385-92.
1. Hospital Nossa Senhora das Graças, Curitiba, PR, Brazil.
2. Hospital de Clínicas da Universidade Federal do Paraná, Curitiba, PR, Brazil.
3. Hospital Erasto Gaertner, Curitiba, PR, Brazil.
Corresponding author: Anne Karoline Groth Rua Padre Anchieta, 2050, Sala 1512, Curitiba, PR, Brazil. Zip Code: 80730-000. E-mail: altinofn@hotmail.com
Article received: December 5, 2018.
Article accepted: April 16, 2019.
Conflicts of interest: none.




 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter