

Original Article - Year 2020 - Volume 35 - Issue 1
Breast reconstruction with implant: creating a pocket with a reverse serratus anterior muscle flap
Reconstrução mamária com implante: confecção de bolsa com retalho reverso de músculo serrátil anterior
ABSTRACT
Introduction: Immediate breast reconstruction plays an important role in the treatment of breast cancer and relatively promotes patients' emotional and physical recovery. It may be difficult to cover the entire prosthesis with a muscle flap in single-stage breast reconstructions based on a permanent implant. This study aimed to present a muscle pocket for the implant using a reverse anterior serratus muscle flap associated with submuscular dissection of the pectoralis major muscle.
Methods: This was a prospective study comprising 61 patients undergoing mastectomy followed by immediate reconstruction (74 reconstructions) with implant and anterior serratus muscle reverse flap associated with submuscular pectoralis dissection between January 2017 and July 2018. In this study, age, adjuvant and neoadjuvant therapies, implant volume, length of hospital stay, follow-up, and complications, including functional deficit and reconstruction failure, were analyzed.
Results: The technique described was used to operate 74 patients with a mean age of 49.2 years. The volume of the implant varied from 200 to 500 cc, and the mean follow-up time was 14.9 months. Complications such as hematoma, suture dehiscence, skin flap necrosis, and implant extrusion were observed in 14 patients (18.9%).
Conclusion: In most cases, breast reconstruction with an anterior serratus muscle reverse flap associated with submuscular dissection of the pectoralis major muscle allows the complete muscle coverage of the implant, reduces the occurrence of major surgical complications, and has a good aesthetic result.
Keywords: Surgery, Plastic; Breast neoplasms; Mastectomy; Reconstruction; Breast implants.
RESUMO
Introdução: A reconstrução mamária imediata desempenha papel importante no tratamento do câncer de mama, permitindo uma recuperação emocional e física, ainda que parcial, das pacientes. Nas reconstruções mamárias em único estágio, baseada em implante permanente, pode ser difícil cobrir toda a prótese com retalho muscular. O objetivo do estudo é apresentar a realização de uma bolsa muscular para implante através do retalho reverso do músculo serrátil anterior associada à dissecção submuscular do músculo peitoral maior.
Métodos: Foi realizado um estudo prospectivo com 61 pacientes submetidas à mastectomia seguida de reconstrução imediata (74 reconstruções) com implante e retalho reverso do músculo serrátil anterior associado à dissecção submuscular do peitoral, entre janeiro de 2017 e julho de 2018. Foram analisados a idade, terapia adjuvante e neoadjuvante, volume do implante, tempo de internação hospitalar, seguimento e complicações, incluindo déficit funcional e falha na reconstrução.
Resultados: 74 pacientes foram operadas pela técnica descrita com idade média de 49,2 anos. O volume do implante variou de 200 a 500cc e o tempo médio de proservação foi de 14,9 meses. 14 pacientes (18,9%) apresentaram complicações como hematoma, deiscência de sutura, necrose de retalho cutâneo e extrusão do implante.
Conclusão: A reconstrução mamária com retalho reverso do músculo serrátil anterior associado à dissecção submuscular do peitoral maior permite, na grande maioria dos casos, cobertura muscular completa do implante, redução de complicações cirúrgicas maiores e bom aspecto estético.
Palavras-chave: Cirurgia plástica; Neoplasias da mama; Mastectomia; Reconstrução; Implante mamário
INTRODUCTION
Immediate breast reconstruction plays an important role in the treatment of breast cancer and relatively promotes patients’ emotional and physical recovery1,2.
Types of breast reconstruction include implant placement and/or use of autologous tissue. The use of permanent implants or expanders is widely accepted and increasingly recommended, specifically with the increased number of skin- and nipple-areolar complex (NAC)-sparing mastectomies3.
It may be difficult to cover the prosthesis with a muscle or fascial flap in single-stage reconstructions based on a permanent implant.
This study presents the technique of creating a muscle pocket for the implant using a reverse anterior serratus muscle flap associated with submuscular dissection of the pectoralis major.
OBJECTIVE
This study aimed to present the technique of creating a muscle pocket with a reverse serratus muscle flap to cover the implant in immediate breast reconstruction.
METHODS
This was a prospective, descriptive, and analytical study following the principles of the Declaration of Helsinki revised in 2000 and Resolution 196/96 of the National Health Council. The study was approved by the research ethics committee of the Felício Rocho Hospital (CAAE 94178618.0.0000.5125) (opinion number 2,947,562). All patients included in this study signed an informed consent form. The authors declare no conflicts of interest, and there were no sources of funding.
A total of 61 patients underwent mastectomy at the Breast Care Clinic of Felício Rocho Hospital (Belo Horizonte/MG, Brazil) between January 2017 and July 2018. Apart from these 61 mastectomies, 13 mastectomies were bilateral, resulting in 74 immediate breast reconstructions with permanent implant. The reconstructions were performed at the Plastic Surgery Clinic of the same institution. The prostheses were placed in a pocket formed by the reverse anterior serratus muscle flap and the submuscular dissection of the pectoralis major.
The inclusion criterion was as follows: patients undergoing skin- or NAC-sparing mastectomy with an indication for immediate unilateral or bilateral reconstruction with permanent implant.
The exclusion criteria were as follows: previous remote mastectomy, inflammatory breast cancer, and patients with large skin resections (with indication for a musculocutaneous flap or expander). Moreover, patients with inadequate postoperative follow-up were excluded in the study.
The studied variables were age, adjuvant and neoadjuvant therapies, implant volume, length of hospital stay, follow-up, and complications, including functional deficit and reconstruction failure.
Functional deficit, mainly winged scapula, was evaluated using the Hoppenfeld test4, where the patient is instructed to stand, flex his/her shoulder at 90°, place his/her hands flat on the wall (shoulders close to his/her hands), and extend his/her elbows by pushing his/her body back. During this test, in the presence of winged scapula, the medial half of the scapula is more evident compared to the unaffected side5.
Reconstruction failure was considered in patients who required reoperation to replace or remove the permanent implant or a rescue operation with a musculocutaneous flap during the follow-up period.
Microsoft Office Excel spreadsheets were used for statistical analysis. The related literature was reviewed using the PubMed and LILACS databases.
Surgical technique
The size of the permanent implant is defined in a preoperative consultation by evaluating breast measurements using plastic shells of predetermined volume (Mamasize®). Intraoperatively, the volume of the removed breast is stipulated using the method of Archimedes6 through the total immersion of the surgical specimen in a container filled with 0.9% saline solution. The overflowing solution is collected in a second container placed immediately below the first one and accurately measured by aspiration using a 60-mL syringe.
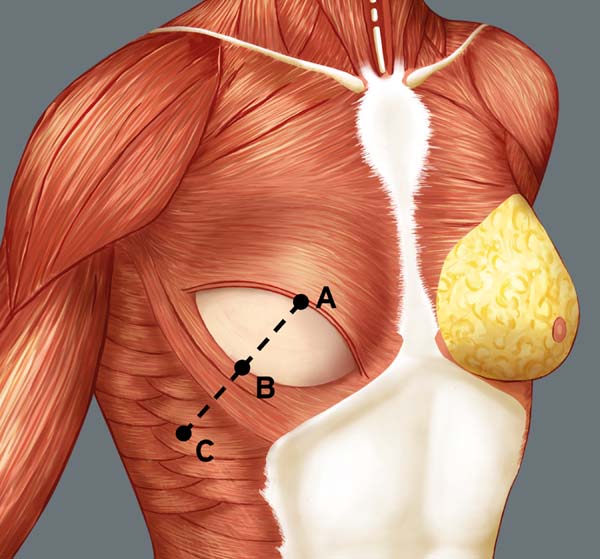
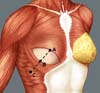
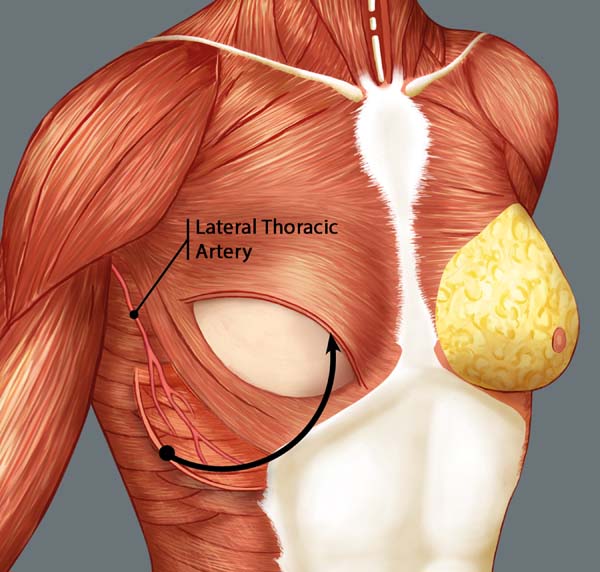
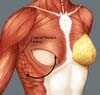
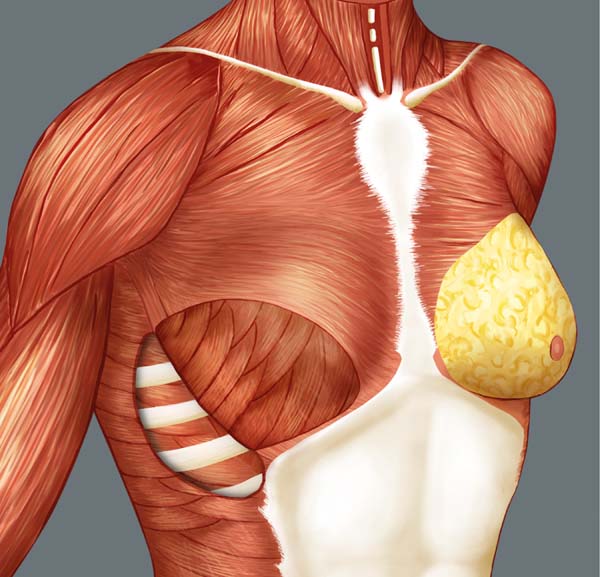
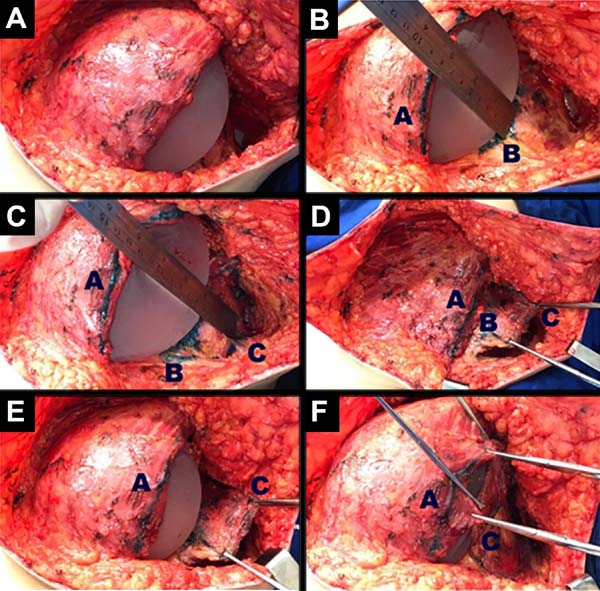
Breast reconstruction begins by detaching the pectoralis major muscle from its lateral margin to its sternal origin using an electrocautery. Inferiorly, the dissection advances at least 2 cm into the sheath of the rectus abdominis muscle, passing the inframammary fold. The permanent implant is placed in a subpectoral pocket (Figure 1), and the pocket with reverse anterior serratus muscle flap is marked. Line A is defined as the lateral margin of the pectoralis major, line B as the base of the permanent implant in the chest wall, and line C as the transfer of the distance between lines A and B. The width of the reverse anterior serratus muscle flap should be adequate for inferolateral muscle coverage of the alloplastic material (Figure 2). Lines A and C are approximated and sutured with separate polyglactin 2 (Vicryl®) stiches as shown in Figures 3 and 4. The procedure ends with the placement of a suction drain, and the surgical wound is sutured by tissue planes (Figure 5).

RESULTS
A total of 61 patients were operated. Apart from these 61 patients, 13 underwent bilateral reconstruction, resulting in 74 breast reconstructions using the technique described (Figures 6 to 8).
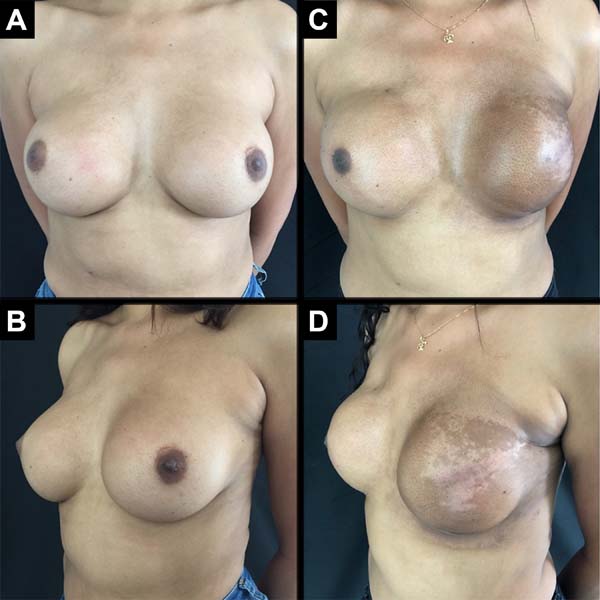
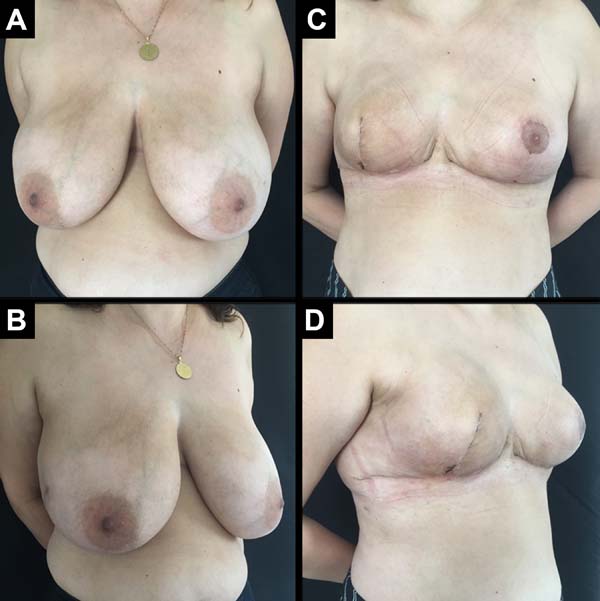
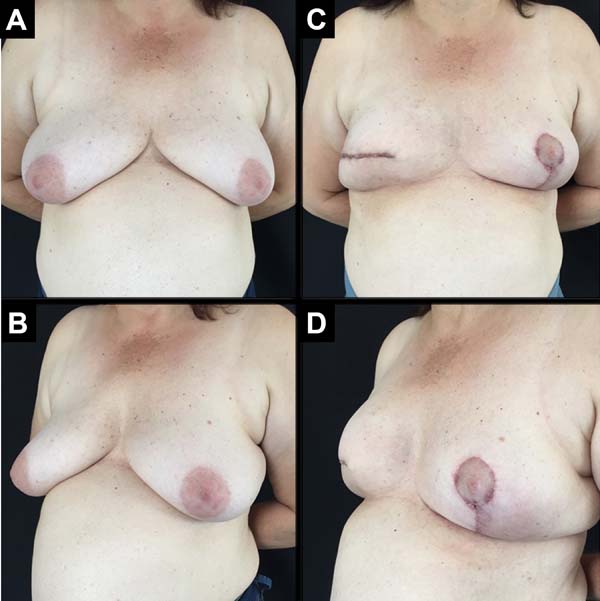
The age of the patients ranged from 32 to 82 years, with a mean age of 49.2 years. The volume of the implants ranged from 200 to 500 cc, with a mean volume of 344.5 cc. Hospital stay was 24 hours for 46 patients (75.4%) and 48 hours for 15 patients (24.6%).
Postoperative follow-up varied from a minimum of 8 months to a maximum of 24 months, with a mean period of 14.9 months.
Thirteen patients (21.3%) had a history of neoadjuvant chemotherapy as a complementary treatment. Of these, nine patients (14.7%) underwent adjuvant radiotherapy, and four (6.5%) underwent adjuvant chemotherapy.
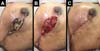
The following complications were observed: persistent seroma after suction drain removal that was treated with aspiration in two (2.7%) patients, hematoma drained in the first 24 hours after surgery in five (6.75%) patients, and wound infection treated with oral antibiotic therapy with improvement in two patients (2.7%). Regarding necrosis, five (6.75%) patients had partial flap necrosis with improvement after conservative treatment with necrosis debridement and implant maintenance (Figure 9), and two (2.7%) patients presented with implant extrusion and removal, followed by a rescue operation with a latissimus dorsi muscle flap.
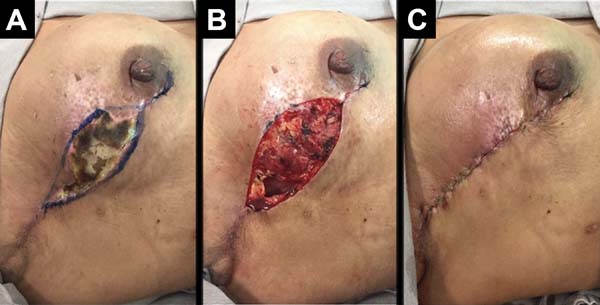
The overall incidence of complications was 18.9% (14 patients). Most of the complications were considered minor, and a total of 2.7% (two patients) of complications were considered major with reconstruction failure. Complaints of severe pain and significant functional limitations during follow-up were not observed.
DISCUSSION
Advancements in breast oncology and complementary treatments and improved screening resulted in increased indications for total skin- and NAC-sparing mastectomies, consequently increasing the number of reconstructions using implants7,8.
Breast reconstruction with implants and immediate reconstruction increased by 11% and 5% per year, respectively. The indication to perform these reconstructions and the choice of technique are individualized, taking into consideration the medical team and the patient9.
The anterior serratus muscle has a jagged outline that is significantly similar to the edge of a saw blade; hence, the term serratus comes from the Latin term serra meaning “saw.” It is located in the lateral posterior side of the chest and originates from the lateral sides of the first to tenth ribs. Its fibers follow the posterior direction and are attached to the anterior face of the medial margin of the scapula, including its lower angle. It has three portions: the first comprises muscle fibers from the first to the second rib, the second portion from the second to the fourth rib, and the third portion from the fifth to the tenth rib. The main function of this muscle is to protrude and rotate the scapula and keep it against the chest wall10,11.
It is mostly innervated by the long thoracic nerve (Bell’s nerve), which originates from the spinal nerve roots (C5 to C7). It starts an upper anteromedial path, passes through the oblique muscle, and crosses the vascular pedicle. Throughout this path, the main nerve trunk has several branches. Thus, long flaps can be obtained by dissecting the anterior serratus muscle, preserving the innervation of the remaining muscle. This prevents the development of a winged scapula11,12.
The anterior serratus muscle flap is classified as group III by Mathes and Nahai (1997)13, with a rich vascularization by the dominant vascular pedicles (branches of the lateral thoracic artery and thoracodorsal artery). It also has collateral vascularization through the lateral perforating branches of the intercostal arteries, which are widely anastomosed with branches of the thoracodorsal artery and form an important and constant source of arterial nutrition13,14.
The use of the anterior serratus muscle in reconstructive surgery is widely described in the literature. It is used as a free flap, a pedicled muscle flap, or a myofascial cutaneous flap15-17. The proposed surgical technique using a reverse anterior serratus muscle flap improves breast reconstruction with implant.
In immediate breast reconstruction with more common regional muscle flaps, the definitive implant is covered by the pectoral muscle, usually in the upper two-thirds. The lower and lateral thirds are unprotected. In most cases, the reverse flap of the anterior serratus muscle allows for a complete muscle coverage of the implant. In some cases, part of the external oblique muscle can be used with the serratus for better implant coverage18. Complete coverage of the prosthesis is important in thin skin flaps. The proposed technique recreates the lateral fold containing the implant and has a good aesthetic result.
Regarding skin- and NAC-sparing mastectomy, the possibility of skin necrosis is always considered and varies in the literature with rates from 0% to 21.6%19-21. Muscle coverage of the implant, specifically in thin skin flaps, reduces the tension on the skin. The implant becomes less noticeable on palpation, and there is no extrusion in cases of dehiscence of the surgical wound or small skin necrosis.
A complete submuscular positioning of the implant can be elevated in the upper pole of the reconstructed breast, with upward displacement of the inframammary fold22. Hence, the dissection in the technique presented advances at least 2 cm into the sheath of the rectus abdominis muscle, passing the inframammary fold.
Both the proposed muscle flap and the acellular dermal matrix (ADM) aim to support the inferolateral part in immediate breast reconstruction and to provide total implant coverage. ADM has the following advantages: it has a short operation time and is an easily performed surgical technique. On the contrary, ADM is considered costly23-25.
A recent meta-analysis suggests that ADM has a higher rate of complications than submuscular reconstruction, such as infection and seroma26.
Breast reconstruction with saline expanders has the following disadvantages: results in multiple returns for gradual expansion, produces pain after expansion, and requires a second operation when a permanent implant is in place, increasing costs27,28.
In this case selection, there were no important functional sequelae, such as winged scapula. The emission of multiple branches by the long thoracic nerve allows an effective innervation of the remaining anterior serratus muscle. The upper portion of the anterior serratus muscle was spared while making the flap, and the function of the trapezius stabilized the scapula.
All patients were followed up by the specialized physiotherapy team at the oncology clinic of the same institution.
Thirteen patients (21.3%) underwent neoadjuvant chemotherapy. Currently available scientific evidence states that immediate breast reconstruction is safe in this group of patients, and the number of postoperative complications does not significantly increase29,30. All patients underwent surgery at least 15 days after the end of complementary therapy.
Adjuvant treatment with radiotherapy is an increasingly recommended practice in breast cancer. It has several oncological benefits, but collateral damage to the chest wall and to the quality of the breast skin negatively affects breast reconstruction, with relatively high complication rates31. A recent study on postoperative morbidity associated with radiotherapy in reconstruction with implants shows a complication rate of 45.3% and a reconstruction failure of 29.4%32. The present study had an incidence of complications less than that reported in the literature: 18.9% for general complications and 2.7% for reconstruction failure. Patients must be properly advised on these possible complications so that shared decisions can be made.
Nine patients (14.7%) underwent adjuvant radiotherapy, and Baker grade III and IV capsular contracture was not identified33. Long-term follow-up of this group of patients and the inclusion of more patients undergoing adjuvant radiotherapy may increase the incidence of this complication.
Although this study has several strengths, it also has the following limitations: this study has a relatively small sample size and a short follow-up period. An increased number of patients and longer follow-up periods can provide more valuable information. Although some patients who require large volume implants and have less developed muscle tissue may experience difficulties in fully covering the implant, this technique can still be performed.
CONCLUSION
The reverse serratus anterior muscle flap is a useful approach in immediate breast reconstruction with implant.
COLLABORATIONS
|
ACMA |
Analysis and/or data interpretation, Conception and design study, Data Curation, Final manuscript approval, Methodology, Project Administration, Realization of operations and/or trials, Supervision, Writing - Original Draft Preparation, Writing - Review & Editing |
|
AFSF |
Analysis and/or data interpretation, Conception and design study, Conceptualization, Final manuscript approval, Investigation, Methodology, Project Administration, Realization of operations and/or trials, Supervision, Validation, Writing - Original Draft Preparation, Writing - Review & Editing |
|
RSR |
Analysis and/or data interpretation, Data Curation, Final manuscript approval, Formal Analysis, Investigation, Writing - Original Draft Preparation, Writing - Review & Editing |
|
NAP |
Analysis and/or data interpretation, Final manuscript approval, Realization of operations and/or trials, Validation, Visualization, Writing - Original Draft Preparation, Writing - Review & Editing |
|
RPLF |
Analysis and/or data interpretation, Final manuscript approval, Realization of operations and/or trials, Validation, Visualization, Writing - Review & Editing |
|
EHP |
Analysis and/or data interpretation, Final manuscript approval, Formal Analysis, Realization of operations and/or trials, Validation, Visualization, Writing - Original Draft Preparation, Writing - Review & Editing |
|
RSOF |
Final manuscript approval, Validation, Visualization, Writing - Review & Editing |
|
JCRRA |
Analysis and/or data interpretation, Final manuscript approval, Supervision, Validation, Visualization, Writing - Review & Editing |
REFERENCES
1. Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet. 1983;26(1):459-62.
2. Stevens LA, McGrath MH, Druss RG, Kister SJ, Gump FE, Forde KA. The psychological impact of immediate breast reconstruction for women with early breast cancer. Plast Reconstr Surg. 1984;73(4):619-28.
3. Cemal Y, Albornoz CR, Disa JJ, McCarthy CM, Mehrara BJ, Pusic AL, et al. A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg. 2013;131(3):320-6.
4. Hoppenfeld S. Propedêutica ortopédica: coluna e extremidades. Rio de Janeiro: Atheneu; 1996.
5. Mastrella AS, Freitas Junior R, Paulinelli RR, Soares LR. Escapula alada pós-linfadenectomia no tratamento do câncer de mama. Rev Bras Cancerol. 2009;55(4):397-404.
6. Webster R, Machado DP, Milani A, Ely PB. Aperfeiçoando a mensuração do volume mamário na reconstrução imediata com expansores permanentes. Rev Bras Cir Plást. 2013;28(1):72-7.
7. Isken T, Onyedi M, Izmirli H, Alagoz S, Katz R. Abdominal fascial flaps for providing total implant coverage in one-stage breast reconstruction: an autologous solution. Aesthetic Plast Surg. 2009;33(6):853-8.
8. Roostaeian J, Pavone L, Da Lio A, Lipa J, Festekjian J, Crisera C. Immediate placement of implants in breast reconstruction: patient selection and outcomes. Plast Reconstr Surg. 2011;127(4):1407-16.
9. Albornoz CR, Bach PB, Mehrara BJ, Disa JJ, Pusic AL, McCarthy CM, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15-23.
10. Cuadros CL, Driscoll CL, Rothkopf DM. The anatomy of the lower serratus anterior muscle: a fresh cadaver study. Plast Reconstr Surg. 1995;95(1):93-7.
11. Vu P, Guedon C, Gehanno P, Andreassian B. Anatomic basis of serratus anterior muscle flap transposition. Surg Radiol Anatom. 1988;10(3):173-85.
12. Moore KL, Dalley AF. Anatomia orientada para a clínica. 5ª ed. Rio de Janeiro: Guanabara Koogan; 2007.
13. Mathes SJ, Nahai F. Reconstructive surgery: principles, anatomy, and technique. New York: Churchill Livingstone; 1997.
14. Yii NW, Cronin K. Vascular anatomy of the serratus anterior muscle. Plast Reconstr Surg. 2005;116(2):680-2.
15. Takayanagi S, Tsukie T. Free serratus anterior muscle and myocutaneous flaps. Ann Plast Surg. 1982;8(4):277-83.
16. Arnold PG, Pairolero PC, Waldorf JC. The serratus anterior muscle: intrathoracic and extrathoracic utilization. Plast Reconstr Surg. 1984;73(2):240-8.
17. Inoue T, Ueda K, Hatoko M, Harashina T. The pedicled extended serratus anterior myocutaneous flap for head and neck reconstruction. Br J Plast Surg. 1991;44(4):259- 65.
18. Tostes ROG, Andrade Júnior JCCG, Silva KDA, Couto AO, Ribeiro GVC, Avelar LET. Reconstrução imediata de mama com prótese de silicone retromuscular: padronização de retalhos musculares. Rev Bras Cir Plást. 2005;20(4):213-9.
19. Carlson GW, Bostwick J, Styblo TM, Moore B, Bried JT, Murray DR, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg. 1997;225(5):570-5.
20. Mosahebi A, Ramakrishnan V, Gittos M, Collier DS. Envelope mastectomy and immediate reconstruction (EMIR), improving outcome without oncological compromise. J Plast Reconstr Aesthet Surg. 2006;59(10):1025-30.
21. Toth BA, Forley BG, Calabria R. Retrospective study of the skin-sparing mastectomy in breast reconstruction. Plast Reconstr Surg. 1999;104(1):77-84.
22. Isken T, Onyedi M, Izmirli H, Alagoz S, Katz R. Abdominal fascial flaps for providing total implant coverage in one-stage breast reconstruction: an autologous solution. Aesthetic Plast Surg. 2009;33(6):853-8.
23. Salzberg CA, Dunavant C, Nocera N. Immediate breast reconstruction using porcine acellular dermal matrix (StratticeTM): long-term outcomes and complications. J Plast Reconstr Aesthet Surg. 2013;66(3):323-8.
24. Nguyen TJ, Carey JN, Wong AK. Use of human acellular dermal matrix in implant-based breast reconstruction: evaluating the evidence. J Plast Reconstr Aesthet Surg. 2011;64(12):1553-61.
25. Vardanian AJ, Clayton JL, Roostaeian J, Shirvanian V, Da Lio A, Lipa JE, et al. Comparison of implant bases immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128(5):403e-10e.
26. Kim JY, Davila AA, Persing S, Connor CM, Jovanovic B, Khan SA, et al. A meta-analysis of human cellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129(1):28-41.
27. Bordoni D, Cadenelli P, Rocco N, Tessone A, Falco G, Magalotti C. Serratus anterior fascia flap versus muscular flap for expander coverage in two-stage breast reconstruction following mastectomy: early post-operative outcomes. Aesthetic Plast Surg. 2017;41(1):26-30.
28. Roostaeian J, Sanchez I, Vardanian A, Herrera F, Galanis C, Da Lio A, et al. Comparison of immediate implant placement versus the staged tissue expander technique in breast reconstruction. Plast Reconstr Surg. 2012;129(6):909e-18e.
29. Cassidy MR, Zabor EC, Stempel M, Mehrara B, Gemignani ML. Does response to neo-adjuvant chemotherapy impact breast reconstruction?. Breast J. 2018;24(4):567-73.
30. Bowen ME, Mone MC, Buys SS, Sheng X, Nelson EW. Surgical outcomes for mastectomy patients receiving neoadjuvant chemotherapy: a propensity-matched analysis. Ann Surg. 2017;265(3):448-56.
31. Momoh AO, Ahmed R, Kelley BP, Aliu O, Kidwell KM, Kozlow JH, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21(1):118-24.
32. Chetta MD, Aliu O, Zhong L, Sears ED, Waljee JF, Chung KC, et al. Reconstruction of the irradiated breast: a national claims-based assessment of postoperative morbidity. Plast Reconstr Surg. 2017;139(4):783-92.
33. Baker Junior JL, Chandler ML, LeVier RR. Occurrence and activity of myofibroblasts in human capsular tissue surrounding mammary implants. Plast Reconstr Surg. 1981;68(6):905-12.
1. Hospital Felício Rocho, Belo Horizonte, MG, Brazil.
2. Instituto de Cirurgia Plástica Avançada, Belo Horizonte, MG, Brazil.
3. Sociedade Brasileira de Cirurgia Plástica, São Paulo, SP, Brazil.
4. Universidade Federal de São Paulo, São Paulo, SP Brazil.
Corresponding author: Augusto César de Melo Almeida Rua Santa Catarina, 996/701, Lourdes, Belo Horizonte, MG, Brazil. Zip Code: 30170084. E-mail: contato@draugustoalmeida.com.br
Article received: June 17, 2019.
Article accepted: October 21, 2019.
Conflicts of interest: none.







 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter