

Review Article - Year 2019 - Volume 34 -
Rivaroxaban for venous thromboembolism prophylaxis in abdominoplasty after massive weight loss: 396 cases
Rivaroxabana para profilaxia de tromboembolismo venoso em abdominoplastia após grande perda ponderal: 396 casos
ABSTRACT
Introduction: Abdominoplasty is one of the most popular aesthetic procedures performed in Brazil. Postbariatric patients present a challenge to the plastic surgeon as not only do they have complex reconstructive challenges but also they have residual medical comorbidities and nutritional deficiencies. A serious and potentially fatal complication of abdominoplasty is venous thromboembolism (VTE). Despite the frequency of this serious complication, the accepted standard methods to prevent VTE in abdominoplasty patients, including chemoprophylaxis, remain controversy.
Objective: To evaluate the author experience with rivaroxaban, for VTE prophylaxis in abdominoplasty patients after massive weight loss.
Methods: A retrospective 396 cases series were conducted. All patients who underwent abdominoplasty after bariatric surgery and received rivaroxaban were included. The prophylactic dose was 10 mg daily for 30 days, beginning 24 hours postoperatively. Patient demographics, comorbidities, type of surgery and complications were recorded.
Results: From July 2015 until July 2018, 396 post bariatric patients (356 women and 40 men) underwent abdominoplasty and received rivaroxaban postoperatively. The mean body mass index prior to their weight loss procedure was 43.8kg/m2 (range, 37.3- 61.9kg/m2) and mean BMI was 27.2kg/m² at the time of the abdominoplasty. Mean patient age was 39.1 years. Only one patient had a symptomatic PTE event. Thirteen patients had a hematoma requiring operative evacuation, and all went on to heal without sequel.
Conclusions: Routine chemoprophylaxis with rivaroxaban for abdominoplasty patients after massive weight loss has a low rate of VTE events. This oral medication is well tolerated and has an acceptable complication profile.
Keywords: Reconstructive surgical procedures; Venous thrombosis; Abdominoplasty; Anticoagulants; Bariatric surgery
RESUMO
Introdução: Abdominoplastia consiste em um dos procedimentos estéticos mais populares
realizados no Brasil. Pacientes pósbariátricos representam um desafio
peculiar ao cirurgião plástico, uma vez que não só requerem reconstruções
complexas, mas também apresentam comorbidades residuais e deficiências
nutricionais. O tromboembolismo venoso (TEV) constitui uma complicação grave
e potencialmente fatal da abdominoplastia. Apesar da pequena frequência
desta complicação, os métodos aceitos como padrões para prevenção de TEV em
pacientes após abdominoplastia, incluindo quimioprofilaxia, permanecem
controversos.
Objetivo: Avaliar a experiência do autor com rivaroxabana para profilaxia de TEV em
pacientes submetidos a abdominoplastia após grande perda ponderal.
Métodos: Uma série de 396 casos foi conduzida retrospectivamente. Todos os pacientes
submetidos à abdominoplastia após cirurgia bariátrica que receberam
rivaroxabana foram incluídos. A dose profilática foi de 10mg por dia. Dados
demográficos, comorbidades, tipo de cirurgia e complicações foram
registrados.
Resultados: 396 casos de pacientes pós-bariátricos (356 mulheres e 40 homens) foram
submetidos à abdominoplastia e receberam rivaroxabana no pós-operatório, de
julho de 2015 a julho de 2018. A média de idade dos pacientes foi de 39,1
anos. O índice de massa corporal médio no momento da abdominoplastia foi de
27,2kg/m². Houve apenas um caso de tromboembolismo venoso (0,25%). Treze
pacientes apresentaram hematoma com necessidade de drenagem.
Conclusões: A quimioprofilaxia de rotina com rivaroxabana para pacientes submetidos à
abdominoplastia após grande perda ponderal revela uma baixa incidência de
TEV. Esta medicação oral é bem tolerada e apresenta um perfil de complicação
aceitável.
Palavras-chave: Procedimentos cirúrgicos reconstrutivos; Trombose venosa; Abdominoplastia; Anticoagulantes; Cirurgia bariátrica
INTRODUCTION
Abdominoplasty is one of the most popular esthetic procedures performed in Brazil. A serious and potentially fatal complication of abdominoplasty is venous thromboembolism (VTE). A systematic review by Hatef et al.1 demonstrated that the rate of VTE was 0.34% for abdominoplasty-only patients, 0.67% for patients undergoing abdominoplasty with a concomitant plastic surgery procedure, and 3.4% for patients undergoing circumferential abdominoplasty. In a single institutional series, VTE was 5.0% in abdominoplasty patients and 7.7% in circumferential abdominoplasty patients.
Despite the relative frequency of this serious complication, opinion regarding the preferred methods to prevent a VTE in abdominoplasty patients, including chemoprophylaxis, remain controversial². The reasons for not incorporating chemoprophylaxis may be the low rate of VTE when patients are properly selected, limited depth and duration of anesthesia, properly positioning of patients, mechanical prophylaxis, and early postoperative ambulation of patients ³.
Additionally, because VTE prophylaxis agents block portions of the coagulation cascade to inhibit blood clot formation, the risk of bleeding complications is theoretically increased. Prophylaxis for venous thromboembolism is recommended for at least 10 days after high-risk surgeries. An inconvenience associated with the use of low-molecular-weight heparin, is the need for continuous daily injections up to 2 weeks, usually when the patient is recovering at home.
New oral regimens (rivaroxaban) could enable shorter hospital stays, with the added benefit of more reliable prophylaxis4. Rivaroxaban (Xarelto) is an oral Factor Xa inhibitor. The medication gained United States Food and Drug Administration (FDA) approval in 2011 for VTE prevention for patients undergoing hip or knee replacement surgery and in 2013 in Brazil4.
At the standard prophylactic doses (10 mg once daily), measuring drug levels is not necessary, and adjustments for weight or creatinine clearance are not required. We present a retrospective experience of 1 single plastic surgeon on the use of rivaroxaban for routine VTE prophylaxis in patients undergoing abdominoplasty after massive weight loss.
METHODS
A retrospective chart review was conducted on 396 patients who received postoperative rivaroxaban (Xarelto) after having undergone an abdominoplasty. All patients had previously undergone bariatric surgery. All procedures were performed under general anesthesia. A dose of 10 mg of rivaroxaban was initiated 24 hours postoperatively. Since this drug was administered in abdominoplasty patients, it was technically an off-label use as the Food and Drug Administration (FDA) has approved rivaroxaban for VTE prophylaxis in hip and knee replacement patients.
As the drug was approved for a related (but not identical) indication, a specific formal written consent regarding its use following abdominoplasty was not obtained. During routine consultation and consent for surgery, the patients were informed regarding the risks and benefits associated with rivaroxaban, and were notified about its off-label use. The study conformed to the World Medical Association Declaration of Helsinki5.
All clinical and operative notes and laboratory data were utilized for data collection. Data on demographics, comorbidities, body mass index (BMI), surgery performed, length of anesthetic use, and postoperative complications were recorded. Patients were included in the analysis if there was a follow-up of at least 90 days.
In the present study, rivaroxaban was routinely administered to all abdominoplasty patients as they were considered high risk based on the procedure type as cited in the American Society of Plastic Surgeons (ASPS) VTE task force recommendations (which utilized the 2005 Caprini scale), and was prescribed for 30 days6. The first dose was given 24 hours post-procedure. Four hours after the procedure, all patients received 40 mg of subcutaneous enoxaparina. We used enoxaparina because nausea and vomiting are common complications after surgery and may compromise absorption of rivaroxaban. Additional standard practices included prophylaxis using perioperative sequential compression devices, early ambulation, and maintaining adequate hydration.
Postoperative pain was managed with 400 mg of celecoxib (12/12 h for 7 days) and 30 mg of codeine (8/8 h for 5 days). Follow-up was done from the time of surgery till 90 days post-operative. Patients smoking at preoperative visits (typically within 4 weeks of surgery) were classified as smokers and were classified as non-smokers if they reported a negative smoking history. Patients on contraceptive medications or hormone replacement therapies in any form (pill, cream, ring, intrauterine device, injection) at the preoperative visit were classified as positive for contraceptive medications and were encouraged to continue usage.
RESULTS
The chart review included 396 patients who underwent abdominoplasty from July 2015 to July 2018.. All patients had previously undergone a Roux-en-Y gastric bypass (338 laparoscopic and 58 open approach). Gender distribution was uneven with a predominance of females: 356 were female and 40 were male.
The ages of the patients ranged from 20 to 62 years. The mean age was 39.1 years. The mean weight at the time of plastic surgery was 71.5 kg (48-145 kg). The mean body mass index (BMI) prior to the weight loss surgery was 43.8 kg/m2 (range, 37.3-61.9 kg/m2) with a mean BMI of 27.2 kg/m2 (20.4-38.3 kg/m2) at the time of the abdominoplasty.
The mean dose of albumin for the patients was 4.0 mg/dl (3.1-4.5 mg/dl). The mean hemoglobin was 12.7 g/dl and the GV was 38.6%. Among the comorbidities, hypothyroidism was the most prevalent, present in 13 patients. Only 2 patients had hypertension and none had diabetes mellitus. The mean dry abdominal flap weight was 2.53 kg (0.9-14.0 kg). The mean surgical time was 134 minutes (105-180) (Table 1).
| Number | Range | |
|---|---|---|
| Total cases | 396 | NA |
| Mean age (years) | 39.1 | 20-62 |
| Gender | NA | |
| Women | 356 | NA |
| Men | 40 | NA |
| BMI | 27.2 kg/m2 | 20.4-38.3 kg/m2 |
| Diabetic patients | 0 | NA |
| Hypothyroidism | 13 | NA |
| Hypertension | 2 | NA |
| Albumin | 4.0 mg/dl | 3.1-4.5 mg/dl |
| Abdominal flap weight | 2.53 kg | 0.9-14.0 kg |
| Surgical time | 134 minutes | 105-180 minutes |
There were other surgeries associated with the abdominoplasty in this series.
There were 260 (65.6%) conventional abdominoplasties and 136 (34.4%) anchor abdominoplasties. Patients were discharged on the first postoperative day in 100% of the cases. Complications occurred in 29 (7.3%) patients: 13 patients had hematomas (3.2%), the most frequent complication. Additional complications included seroma formation with needle aspiration (n = 4.1%), 2 local infections (infected seroma and cellulitis) and 6 cases of tissue necrosis of small extension.
All cases of hematoma were operated in an outpatient setting without hospitalization. Two patients who were allowed oral diet developed intestinal obstruction between the second and third week. One case was clinically reversed, while the other required laparotomy with flange release of the whole-anastomosis. Both patients had uneventful sequelae.. There was no case of deep vein thrombosis (VTE). One patient had a severe bronchospasm 24 hours after surgery, but pulmonary thrombo-embolism (PTE) was discarded.
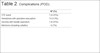
Out of the 396 patients who received postoperative rivaroxaban, 1 patient (0.25%) had a PTE (Table 2). The patient was a 47-year-old woman with a BMI of 28, who had undergone a conventional abdominoplasty. An ultrasound did not reveal a VTE but the CT scan revealed a moderate-large clot burden affecting left pulmonary trunk and confirmed a PTE. She was prescribed a therapeutic dose of enoxaparin, which was substituted with a therapeutic dose of rivaroxaban (20 mg daily) for PTE treatment. She had an uneventful recovered.
DISCUSSION
The prevention of VTE is a high priority in esthetic surgery and has occupied an increasingly large space in all medical specialties; countless protocols can be found in literature. Although abdominoplasty is the most common esthetic procedure associated with VTE, the mechanisms for the development of VTE associated with this procedure are unclear7.
The term “venous thromboembolism” refers to a spectrum of diseases that include deep venous thrombosis and pulmonary embolism. Pulmonary embolism is considered to be a preventable cause of hospital death. Development of thromboembolism involves the following stages: (1) venous stasis, (2) damage to vascular endothelium, and (3) hypercoagulability. During surgery, all these stages are exaggerated. The intraoperative immobilization and the precoagulant state after surgical vascular injury toward the block of the surgical bleeding also act to decrease fibrinolytic activity.
Grazer & Goldwyn8 reported a 1.1% incidence of deep venous thrombosis and a 0.8% incidence of pulmonary embolism in abdominoplasty. Similarly, Hester et al. found that when abdominoplasty was combined with other surgical procedures, the incidence of pulmonary embolism was significantly greater.
A thorough patient history is essential to detect and determine risk. In our casuistic, we have the risk factors of oral contraceptive use, hormone replacement therapy, and a large number of previously obese (post bariatric surgery) patients.
Ideally, chemoprophylaxis should be (1) effective at preventing VTE, (2) associated with a low risk of adverse events such as hematoma formation, (3) inexpensive, (4) without the need for monitoring levels or adjusting doses based on weight or creatinine, (5) easily administered and tolerated by the patient, and (6) administered as part of patient protocol without consulting a risk-stratification scale.
Rivaroxaban is an orally bioavailable factor Xa inhibitor that selectively blocks the active site of factor Xa and does not require a cofactor (such as Anti- thrombin III) for activity. Rivaroxaban was approved for use in several countries, by the European Medicine Agency in 2008, and by the FDA Therapeutics and Clinical Risk Management in 20159.
Activation of factor X to factor Xa (FXa) via the intrinsic and extrinsic pathways plays a central role in the cascade of blood. The maximum concentrations of rivaroxaban appear 2 to 4 hours after tablet intake. The pharmacokinetics of rivaroxaban were not affected by drugs which alter gastric pH.
Administration of rivaroxaban via a method that could deposit drug directly into the proximal small intestine (e.g., feeding tube) should be avoided as it can result in reduced absorption and drug-related exposure. The effect lasts 8 to 12 hours; however, factor Xa activity does not return to normal within 24 hours, so once- daily dosing is possible10.
Rivaroxaban has been demonstrated to be effective for VTE prophylaxis in patients undergoing hip and knee replacement (initial FDA indications), and bleeding rates were found to be similar to those with enoxaparin. The standard duration of therapy is 14 days after knee replacement or 35 days after hip replacement11,12. Rivaroxaban demonstrated cost saving in patients with total hip and total knee replacements when compared to enoxaparin13.
The purpose of this article was to demonstrate that rivaroxaban is a safe medication for thromboprophylaxis in patients undergoing abdominoplasty after massive weight loss. After addition of rivaroxaban, all patients used elastic compression stockings after surgery continuously for 1 month and were ambulatory on the first day after surgery.
In this paper, the abdominoplasty patients included had an average age of were 39.1 years, average BMI was 27.2, and average operative time was 134 minutes. This is not the first published study on rivaroxaban in abdominoplasty patients. Dini et al.14 evaluated rivaroxaban for postoperative VTE prophylaxis in abdominoplasty patients. In a prospective, randomized, double-blinded, placebo controlled study, 40 patients considered high risk were randomized to receive either rivaroxaban 10 mg or placebo daily for 10 days. The drug was first administered 8 hours after surgery. After 27 surgeries, the study was stopped due to a high complication rate; it was noted that all 8 hematomas up to that point had occurred in the study (rivaroxaban) group. It is possible that early initiation of rivaroxaban could have been the cause.
In our study, rivaroxaban was initiated 24 hours after surgery. We preferred to use the subcutaneous enoxaparina 40 mg 4 hours after surgery, instead of rivaroxaban 10 mg, because nausea and vomiting are common after the surgery and may compromise the absorption of rivaroxaban.
No patient developed a VTE and 1 patient who developed a PTE (0.25%) in this study was consistent with the incidence in literature. Plastic surgeons could be interested in the fact that the patient developed symptoms of PTE 16 days after surgery, and surgeons used prophylaxis at most 14 days. Hunstad et al.15 published that 1 patient developed VTE (0.24%) in a multicenter study with 132 patients who underwent abdominoplasty and received rivaroxaban postoperatively, similar to our study. Hustand et al. administrated 10 mg of rivaroxaban for 7 days, which is not sufficient in our opinion as most VTE events occur 10 days after surgery.
Regarding hematomas, Stewart et al.16 published their experience of 278 abdominoplasty patients and reported a 3% hematoma rate. Out of 396 patients in this study, hematoma formation requiring operative evacuation in 13 patients (3.2%) is acceptable, with all hematomas at the abdominal site. When compared to reoperative hematoma rates in the plastic surgery population, our use of rivaroxaban is consistent with previously published data.
Studying 3681 patients who underwent a wide variety of surgical procedures, Pannucci et al.17 reported an overall reoperative hematoma rate of 2.65% when enoxaparin was not administered and 3.38% when enoxaparin was administered (not significant) in a study of 1567 patients at moderate to high risk for VTE events according to the 2005 Caprini scale and 2114 matched historical control patients.
Regarding the 2 patients who developed intestinal obstruction (0.5%) between the second and third week of oral diet, we believe that the higher intra-abdominal pressure (owing the rectus muscle plication), associated with constipation could be an explanation. Champion & Williams18,in 2003, demonstrated an overall incidence of intestinal obstruction of 1.8% in a large series of laparoscopic gastric bypass patients.
A potential limitation of our study is that since this study reports on a patient series without a comparison group, we cannot make conclusions regarding superiority or inferiority of rivaroxaban use for abdominoplasty patients with respect to VTE prevention or complication rates.
The strength of this study is our inclusion of all consecutive patients during our study period who took rivaroxaban with adequate follow-up for relevant hematologic and surgical complications. In our experience, rivaroxaban has a low rate of adverse events such as hematoma formation and is well tolerated by patients without a large burden of cost.
The absence of symptomatic deep vein thrombosis and only one case of pulmonary embolism do not allow any effective conclusion since the statistical sample was small. However, this should open a fresh perspective for future studies in the national and worldwide plastic surgery societies where large samples can be obtained to prove the safety (or not) of rivaroxaban in deep vein thrombosis prophylaxis for patients undergoing abdominoplasty after massive weight loss.
CONCLUSIONS
Routine chemoprophylaxis with rivaroxaban for abdominoplasty after massive weight loss patients has a low rate of VTE events. This oral medication is well tolerated and has an acceptable complication profile.
COLLABORATIONS
|
GBR |
Analysis and/or data interpretation, conception and design study, data curation, final manuscript approval, formal analysis, methodology, project administration, realization of operations and/or trials, supervision, visualization, writing - review & editing. |
|
BFB |
Final manuscript approval, formal analysis, writing - review & editing. |
|
RSF |
Get out of criticism of your content. |
REFERENCES
1. Hatef DA, Kenkel JM, Nguyen MQ, Farkas JP, Abtahi F, Rohrich RJ, et al. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122(1):269-79.
2. Pannucci CJ, Dreszer G, Wachtman CF, Bailey SH, Portschy PR, Hamill JB, et al. Postoperative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients. Plast Reconstr Surg. 2011;128(5):1093-103.
3. Pannucci CJ. Reply: reducing venous thromboembolism risk without chemoprophylaxis. Plast Reconstr Surg. 2013;131(3):451e-2e.
4. Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al.; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776-86.
5. World Medical Association (WMA). Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. [cited 2015 Apr 5]. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
6. Murphy RX Jr, Alderman A, Gutowski K, Kerrigan C, Rosolowski K, Schechter L, et al. Evidence-based practices for thromboembolism prevention: summary of the ASPS Venous Thromboembolism Task Force Report. Plast Reconstr Surg. 2012;130(1):168e-75e.
7. Hatef DA, Trussler AP, Kenkel JM. Procedural risk for venous thromboembolism in abdominal contouring surgery: a systematic review of the literature. Plast Reconstr Surg. 2010;125(1):352-62.
8. Grazer FM, Goldwyn RM. Abdominoplasty assessed by survey, with emphasis on complications. Plast Reconstr Surg. 1977;59(4):513-7.
9. Munson CF, Reid AJ. Novel oral anticoagulants in plastic surgery. J Plast Reconstr Aesthet Surg. 2016;69(5):585-93.
10. Samama MM. The mechanism of action of rivaroxaban--an oral, direct Factor Xa inhibitor--compared with other anticoagulants. Thromb Res. 2011;127(6):497-504.
11. Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al.; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765-75.
12. Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al.; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776-86.
13. Duran A, Sengupta N, Diamantopoulos A, Forster F, Kwong L, Lees M. Cost effectiveness of rivaroxaban versus enoxaparin for prevention of post-surgical venous thromboembolism from a U.S. payer’s perspective. Pharmacoeconomics. 2012;30(2):87-101.
14. Dini GM, Ferreira MC, Albuquerque LG, Ferreira LM. How safe is thromboprophylaxis in abdominoplasty? Plast Reconstr Surg. 2012;130(6):851e-7e.
15. Hunstad JP, Krochmal DJ, Flugstad NA, Kortesis BG, Augenstein AC, Culbertson GR. Rivaroxaban for Venous Thromboembolism Prophylaxis in Abdominoplasty: A Multicenter Experience. Aesthet Surg J. 2016;36(1):60-6.
16. Stewart KJ, Stewart DA, Coghlan B, Harrison DH, Jones BM, Waterhouse N. Complications of 278 consecutive abdominoplasties. J Plast Reconstr Aesthet Surg. 2006;59(11):1152-5.
17. Pannucci CJ, Wachtman CF, Dreszer G, Bailey SH, Portschy PR, Hamill JB, et al. The effect of postoperative enoxaparin on risk for reoperative hematoma. Plast Reconstr Surg. 2012;129(1):160-8.
18. Champion JK, Williams M. Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13(4):596-600.
1. Medicina Privada, Curitiba, PR,
Brazil.
2. Universidade Federal do Paraná, Curitiba, PR,
Brazil.
Corresponding author: Guilherme Berto Roça, Rua Emiliano Perneta 390, 7º ANDAR, CJ 707, Centro, Curitib, PR, Brazil. Zip Code 80420-080 . E-mail: guiberto@hotmail.com
Article received: October 29, 2018.
Article accepted: February 10, 2019.
Conflicts of interest: none.



 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter