

Original Article - Year 2019 - Volume 34 -
Breast reconstructions: a 16-year retrospective study
Reconstruções mamárias: estudo retrospectivo de 16 anos
ABSTRACT
Introduction: Breast cancer is the second most common type of cancer among women in Brazil. An estimated 59,700 new cases of breast cancer were reported in the 2018-2019 biennium. Breast reconstruction is a safe procedure, and various surgical procedures have been described, including conservative techniques and use of neighborhood flaps, alloplastic materials, and pedicled and microsurgical myocutaneous flaps. The objective of this study was to analyze cases of breast reconstruction after mastectomy for breast cancer performed over a period of 16 years.
Methods: We reviewed the medical records of patients who underwent breast reconstruction after mastectomy for breast cancer between January 2002 and December 2017.
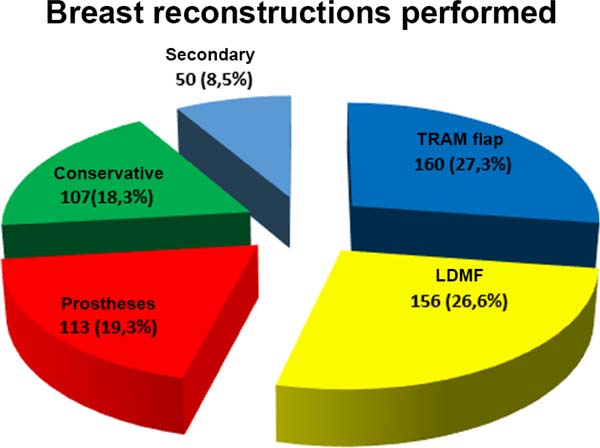
Results: Within the study period, 586 patients underwent breast reconstruction. Breast reconstruction was performed with a transverse rectus abdominis myocutaneous muscle (TRAM) flap in 160 patients, with conservative techniques in 107 patients, with a latissimus dorsi myocutaneous flap (LDMF) in 156 patients, with prostheses in 113 patients, and secondarily in 50 patients. Before October 2007, the proportion of patients who underwent bilateral surgeries with a TRAM flap and LDMF was 30%, and since then, the percentage has increased to 83.3%. One or more types of complications occurred in 203 patients (34.64%) who underwent surgery, with a total of 335 complications . Most outpatient complications did not require surgical reassessment.
Conclusion: The incidence of bilateral surgery increased, which is attributed to the increase in the number of prophylactic mastectomies in the contralateral breast and in the number of reconstructions using a LDMF compared with that using a TRAM flap. A significant increase in the number of reconstructions with silicone implants was also observed.
Keywords: Breast cancer; Breast; Surgical flap; Breast implant; Reconstructive surgical procedure
RESUMO
Introdução: Câncer de mama é o segundo tipo mais comum de câncer entre mulheres no
Brasil. Estimam-se 59.700 casos novos de câncer de mama para o biênio
2018-2019. Reconstrução mamária é um procedimento seguro e vários
procedimentos cirúrgicos são descritos para sua realização: técnicas
conservadoras, retalhos de vizinhança, materiais aloplásticos, retalhos
miocutâneos pediculados e microcirúrgicos. O objetivo deste estudo foi
analisar os casos de reconstrução mamária pós-mastectomia por câncer de
mama, realizados em um período de 16 anos.
Métodos: Foi realizada revisão de prontuários de pacientes submetidas à reconstrução
mamária pós-mastectomia por câncer de mama, no período de janeiro de 2002 a
dezembro de 2017.
Resultados: No período analisado, 586 pacientes foram submetidas à reconstrução mamária.
Em 160 pacientes a reconstrução mamária foi realizada com retalho miocutâneo
do músculo retoabdominal (TRAM), 107 com técnicas conservadoras, 156 com
retalho miocutâneo do músculo grande dorsal (RGD), 113 com próteses e 50
secundárias. Previamente a outubro de 2007, a porcentagem de pacientes
submetidas a cirurgias bilaterais, somando-se TRAM e RGD, era de 30%, e a
partir desse período a porcentagem passou para 83,3%. Houve algum tipo de
complicação ou intercorrência em 203 (34,64%) pacientes operadas,
totalizando de 335 complicações. Grande maioria apresentou intercorrências
tratadas ambulatorialmente sem necessidade de reabordagem cirúrgica.
Conclusão: Houve aumento da incidência de cirurgias bilaterais, fato atribuído ao
aumento das mastectomias profiláticas na mama contralateral e aumento do
número de reconstruções utilizando RGD em comparação com o TRAM, bem como o
aumento significativo das reconstruções com implante de silicone.
Palavras-chave: Neoplasias da mama; Mama; Retalhos cirúrgicos; Implante mamário; Procedimentos cirúrgicos reconstrutivos
INTRODUCTION
According to the National Cancer Institute, breast cancer is the second most common type of cancer, after non-melanoma skin cancer, among women in Brazil and in the world. An estimated 59,700 new cases of breast cancer occurred each year of the 2018-2019 biennium in Brazil, with an estimated risk of 56.33 cases per 100,000 women. The disease accounts for approximately 25.2% of new cancer cases recorded worldwide1.
Multiple factors are involved in the etiology of breast cancer, including age at first menstruation of <12 years; menopause after 55 years; women who have never become pregnant or never had children (nulliparity); first pregnancy after 30 years; use of certain contraceptives and hormone replacement therapy in menopause, especially for prolonged periods; exposure to ionizing radiation; consumption of alcoholic beverages; hypercaloric diets; sedentary lifestyle; and genetic predisposition (due to genetically transmitted mutations in certain transmitted genes, mainly the two high-risk genes, BRCA1 and BRCA2)1.
Owing to its high incidence, this cancer is a major concern, especially because of the psychological and social impacts on women’s health. In an attempt to reduce the negative feelings triggered by the disease and mastectomy, to improve self-esteem, and to make up for the lack of breasts, many women opt for surgical reconstruction. It is a safe procedure that does not increase the risk of recurrence or interfere in the detection of the disease, and does not lead to delays of adjuvant therapies. The effectiveness of several surgical procedures that use conservative techniques, neighborhood flaps, alloplastic materials, and pedicled and microsurgical myocutaneous flaps have been described2,3.
With the emergence of genetic testing for BRCA1 and BRCA2 for risk stratification, the frequency of contralateral prophylactic mastectomy is increasing considerably in patients who had undergone mastectomy for unilateral cancer. Besides genetic tests, other factors can contribute to the implementation of prophylactic mastectomy, including multiple mammographic changes, multicentric precursor lesions, difficulty in screening due to dense breasts, and high anxiety of patients4.
OBJECTIVE
The objective of this study was to analyze the cases of breast reconstruction after mastectomy for breast cancer, performed over a period of 16 years at the author’s private clinic, by identifying the age of patients, types of surgery performed (technique used and laterality), complications, presence of risk factors of complications, and evolution of the procedures over time.
METHODS
This retrospective longitudinal observational study was conducted by reviewing the medical records of patients who underwent breast reconstruction after mastectomy for breast cancer between January 2002 and December 2017. The following data were collected: age, type of breast reconstruction performed (technique used and whether unilateral or bilateral), and postoperative complications. Data on the presence of risk factors of complications (comorbidities, smoking, obesity, and radiotherapy) were also collected in the patients’ charts from October 2007.
All the surgeries were performed by the same plastic surgeon in 5 hospitals located in the city of Brasilia (DF).
This research project followed the legal procedures according to resolution 196/96 of the National Health Council regarding research involving human beings and was conducted in accordance with the principles of the Declaration of Helsinki.
RESULTS
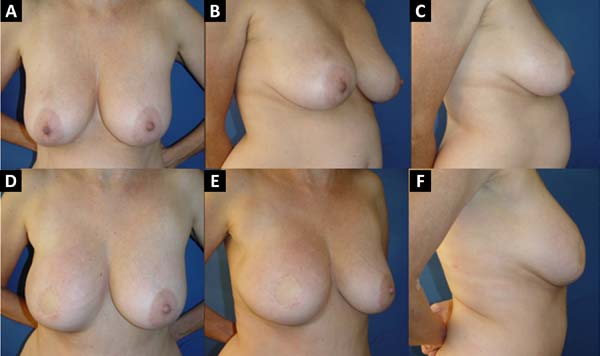
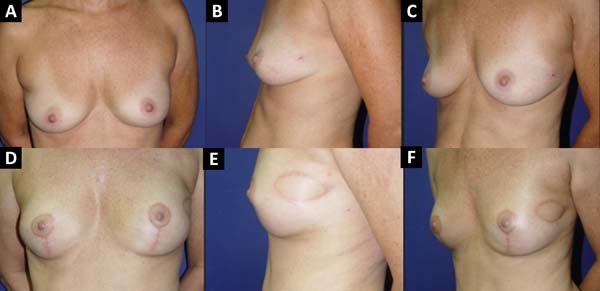
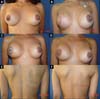
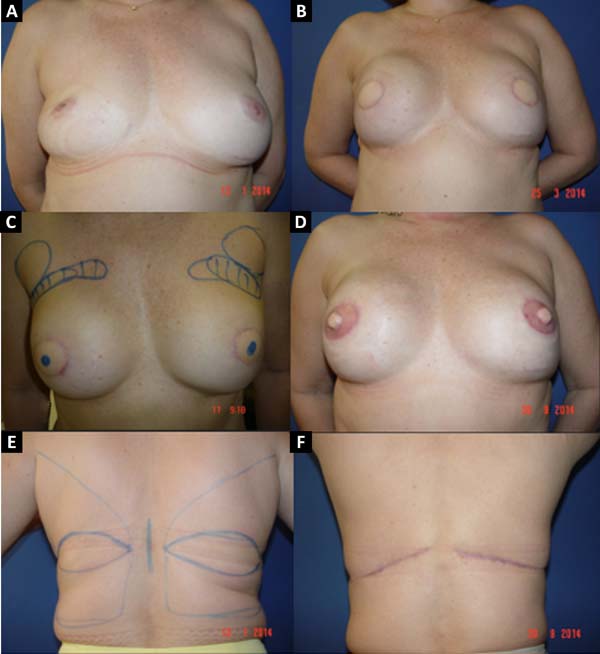
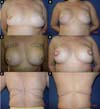
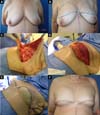
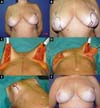
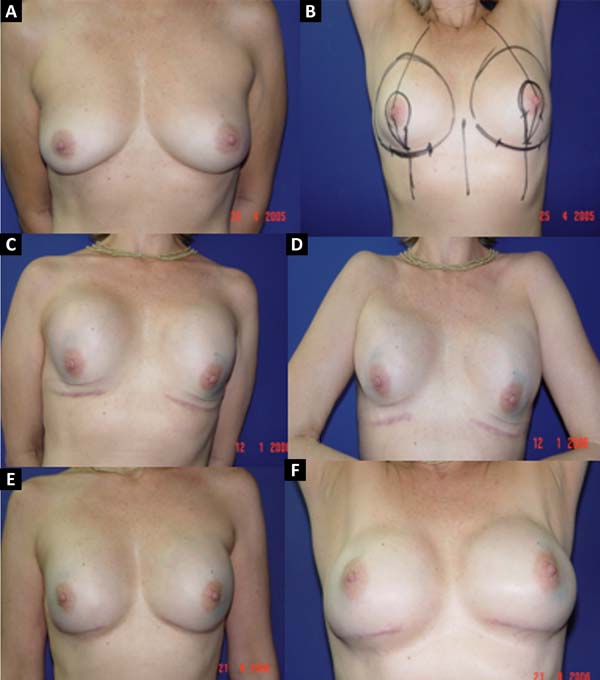
From January 2002 to December 2017, 586 mastectomized breast cancer patients underwent breast reconstruction. The mean age of the patients was 54.37 years, ranging from 27 to 80 years. Breast reconstruction was performed with a transverse rectus abdominis myocutaneous (TRAM) flap in 160 patients, with conservative techniques (local and neighborhood flaps) in 107, with a latissimus dorsi myocutaneous flap (LDMF) in 156, with prostheses in 113 (16 with a larger pectoral muscle flap extended with a lower pedicle dermofat flap and 41 with superior and subcutaneous flaps mixed lower a subcutaneous flap covering the implant), and secondarily in 50 (those that required a new reconstruction due to failure of the first procedure; Figures 1 to 8).
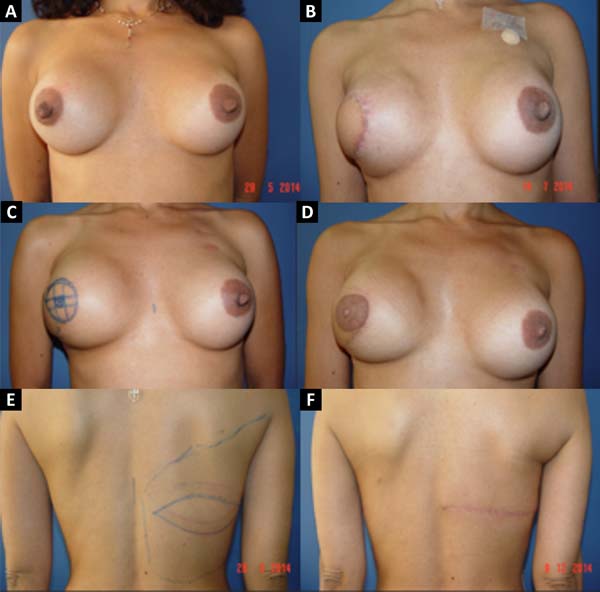
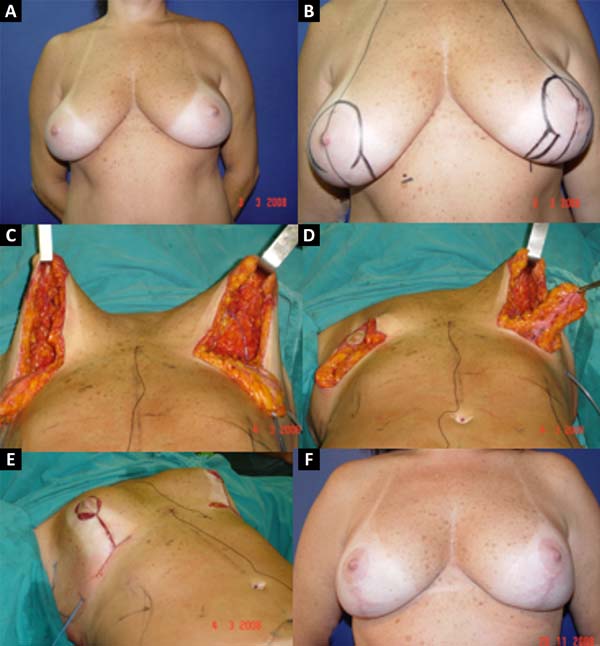
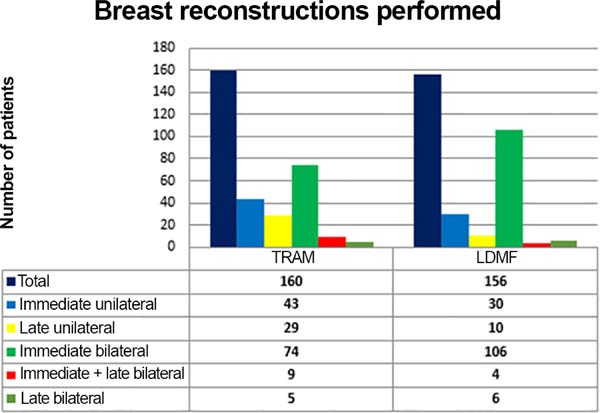
The TRAM procedures performed were unilateral in 72 patients (43 immediate and 29 late) and bilateral in 88 (74 immediate, 5 late, and 9 immediate on one side and late on the other). The LDMF procedures performed were unilateral in 40 patients (30 immediate and 10 late) and bilateral in 116 patients (106 immediate, 6 late, and 4 immediate on one side and late on the other; Figure 9).
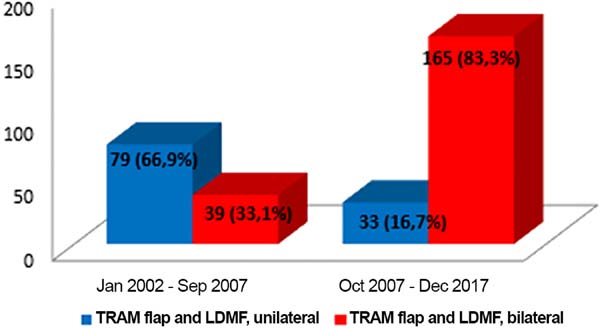
Before October 2007, the percentage of patients who underwent bilateral surgeries, including a TRAM flap and LDMF, was 30%, which increased to 83.3% from then on (Figure 10). The reconstructions were performed with a TRAM flap and LDMF flap, respectively, in 32% and 12% of the patients from 2002 to 2006; in 25.73% and 25.14% from 2007 to 2011; and in 16.45% and 43.67% from 2012 to 2017. During the whole study period, 64.55% and 35.44% of the patients respectively underwent bilateral and unilateral breast reconstructions with a TRAM flap and LDMF.
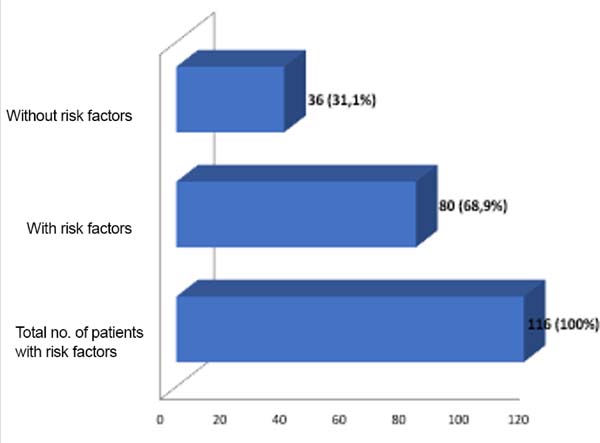
One or more types of complications occurred in 203 patients (34.64%) who underwent operation, with a total of 335 complications (Table 1) and some patients presented with more than one type of complication. Most patients presented complications that were treated on an outpatient basis without the need for surgical review. The analysis of risk factors in the last 293 patients who underwent operation (from October 2007 to December 2017) revealed that 35 patients had obesity (body mass index [BMI], >30 kg/m2), 29 were smokers, 32 had received radiotherapy, and 111 presented comorbidities (diabetes, hypertension, and hypothyroidism). In this group, 116 patients (39.59%) had some complications, of whom 80 (68.9%) had at least one risk factor (Figure 11). Of the 29 smokers, 58.62% presented complications.
| Complications | n | % |
|---|---|---|
| Seroma | 84 | 25.07 |
| Necrosis of the remaining breast skin | 47 | 14.0 |
| Liponecrosis | 37 | 11.0 |
| Dehiscence | 33 | 9.85 |
| Partial flap necrosis | 31 | 9.25 |
| Hematoma | 20 | 5.97 |
| Infection | 19 | 5.67 |
| Capsular contracture (including postoperative radiotherapy) | 18 | 5.40 |
| Partial necrosis of the nipple-areola complex (NAC) | 9 | 2.70 |
| Necrosis of the navel | 8 | 2.40 |
| Abdominal bulging | 8 | 2.40 |
| Mondor's disease | 7 | 2.09 |
| Atelectasis | 5 | 1.50 |
| Pulmonary thromboembolism | 3 | 0.90 |
| Asymmetry | 2 | 0.60 |
| Deep venous thrombosis | 1 | 0.30 |
| Acute renal failure | 1 | 0.30 |
| Obstructive acute abdomen | 1 | 0.30 |
| Pneumonitis | 1 | 0.30 |
| Total | 335 | 100 |
DISCUSSION
Breast reconstruction is an essential part of breast cancer treatment. Several breast reconstruction techniques are available, and the choice of the best technique is influenced by several factors related to oncological treatment such as staging, the need for radiotherapy, the type of mastectomy, and laterality (unilateral or bilateral), and patient-related factors such as BMI, comorbidities, presence of donor areas for autologous reconstruction, preference, expectation in relation to the result, and lifestyle 5. The choice of the technique to be used must be specific to the patient and in accordance with the experience of the plastic surgeon.
In the last 10 years of the study period, an increase in the number of bilateral surgeries performed was observed, although the disease was unilateral, due to the interest of patients to undergo contralateral prophylactic surgery. With the emergence of genetic testing for risk stratification, the frequency of contralateral prophylactic mastectomy is increasing exponentially.
In addition to the genetic tests, other factors can contribute to the implementation of prophylactic mastectomy, including multiple mammographic changes, multicentric precursor lesions, difficulty in screening due to dense breasts, and high patient anxiety6.
In the present study, from 2002 to September 2007, 30% of reconstructions with a TRAM flap and LDMF were bilateral, and this rate was 83.33% from October 2007 to December 2017. From the moment of the treatment of the primary cancer, >10% of women treated will subsequently develop cancer in the contralateral breast, which may emerge up to 30 years after the initial treatment7.
The efficacy of prophylactic mastectomy depends on the ability to remove a large part of the breast tissue, leaving the flap with a minimum viable thickness. The development of cancer in the residual tissue has been reported with an incidence ranging from 1% to 9%8. The effectiveness of the contralateral prophylactic mastectomy in patients treated for breast cancer is estimated at 96%9. In the study of Hartmann et al.6, the reduction in the risk of developing breast cancer in patients who undergo prophylactic mastectomy was close to 90%.
Secondary or rescue reconstruction, defined as complete revision of a previous reconstruction in the event of unsatisfactory outcome or failure of the surgery10, was performed in 50 patients. In 88% of the cases, a myocutaneous flap, TRAM flap, and LDMF were used, which demonstrates that these flaps are well indicated in these cases, as they provide healthy and well-vascularized tissue to a previously manipulated area. Furthermore, on the basis of the numbers shown, over the years, LDMF has been increasingly used in reconstructions in comparison with the TRAM flap.
Treatment of breast cancer used to be mutilating, involving large surgeries that remove large amounts of skin and even muscle. Therefore, the use of TRAM flaps is important in reconstructions that require a considerable volume of tissue to cover the defect of the mastectomy, provided that they have sufficient abdominal adipose tissue9.
With the development of skin-saving mastectomy and the increase in the number of immediate reconstructions performed, in addition to the improvement of the quality of silicone implants, the use of breast reconstruction with a TRAM flap, a surgery of higher morbidity and with disadvantages such as abdominal hernias and bulging, has decreased, and reconstructions with a LDMF and direct implant have increased9.
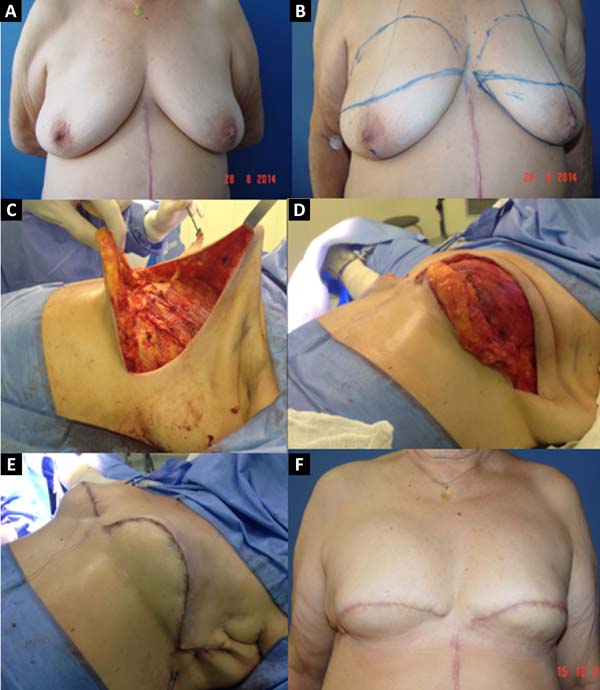
In the 586 patients who underwent breast reconstruction, the extended pectoralis major flap with an inferior dermofat pedicle covering the silicone implant was used in 16 patients (2.73%). This flap is used to lengthen the submuscular pocket. This flap can only be used after joint analysis by the mastologist because it retains a small amount of breast tissue of the lower quadrants. After the concordance of the mastologist to retain this tissue, the location of the tumor, the presence of microcalcifications or other diseases in this region, and the nutritional viability of the flap should be analyzed.
The technique described by Cosac et al.4 provides for an appropriate pocket for the placement of the implant, without excessive tension, with alleviation of projection in the upper and lower poles, and with lower tendency for cephalic migration of the implant. In addition, it presents a minimal risk of prosthesis extrusion, in cases of necrosis or dehiscence, due to the interposition of tissue between the implant and the skin. Besides the excellent aesthetic results, use of the proposed flap does not involve donor site morbidity, prolonged recovery, muscle weakness, nor the use of grafts, being an alternative technique in selected patients4.
In 41 patients (6.99%), breast reconstruction was performed with a prosthesis pocket with a superior retropectoral flap and lower mixed subcutaneous cavity. This technique, described by Cosac et al.9, involves making an arcuate incision in the breast, extending from the lateral end to the medial part of the submammary sulcus. The entire lower base of the breast skin is kept intact, and the dermofat flap should have a minimum thickness of 1.5 to 2 cm. After mastectomy, the plastic surgeon constructs the flap of the pectoralis major muscle, and the implant, after its inclusion, is covered by this muscle in its upper two-thirds and by the dermofat flap in its lower one-third.
This technique results in a single arched scar, which leads to a less stigmatizing aspect. Patient recovery is rapid, with a lower perception of postoperative pain. One of the great advantages of this technique is the presence of a thinner flap, without glandular tissue, which favors greater oncological safety9.
The rate of complications observed in the present study (34.64%) is in agreement with the reported data11. Complications range from 15% to 45%, according to the type of surgery performed, and increase with risk factors (obesity, smoking, comorbidities, and radiotherapy)4, as observed in this study. Not all complications compromise the final result or require new interventions such as seroma, which is responsible for 25.07% of the complications, in patients with outpatient or expectant treatment, rarely implying any permanent complications.
The presence of smoking is associated with a significant increase in the incidence rates of flap necrosis and other complications11. In the group of the last 293 reconstructions studied, the rate of complications in smokers was 58.62%, which is significantly higher than the overall complication rate of 33.41%.
CONCLUSION
The individualization of patients is key to the success of breast reconstruction. Each reconstructive technique has its indications, advantages, and limitations, which should be widely discussed with the patient to obtain the best possible result. The choice of technique also depends on the surgeon’s experience and relationship with the mastology team. We emphasize the need for a multidisciplinary, tuned, well-trained, and qualified surgical team, each working in their area of expertise but synergistically to achieve the best possible surgical outcome.
In this study, an increase in the number of bilateral surgeries performed was observed in recent years, which is attributed to the increase in the number of prophylactic mastectomies performed in contralateral breasts, which can reduce the risk of cancer and facilitate establishment of breast symmetry, with more harmonious results. In recent years, we also observed an increase in the number of reconstructions using the myocutaneous flap of the latissimus dorsi muscle compared with that with the transverse rectus abdominis myocutaneous flap, and a significant increase in the number of reconstructions with silicone implants.
The techniques used in breast reconstruction are effective and safe alternatives, with acceptable complication rates. The presence of risk factors such as obesity, smoking, comorbidities, and radiotherapy, resulted in a higher rate of complications. The surgeon should be aware of all the details, including a well-performed preoperative assessment, correct indication for surgery, and rigorous postoperative follow-up.
COLLABORATIONS
|
OMC |
Analysis and/or data interpretation, final manuscript approval, realization of operations and/or trials. |
|
ACC |
Analysis and/or data interpretation, conception and design study, data curation, writing - review & editing. |
|
RCSD |
Analysis and/or data interpretation. |
|
RSCC |
Analysis and/or data interpretation. |
|
SVS |
Analysis and/or data interpretation. |
|
AAD |
Analysis and/or data interpretation. |
|
JPPCF |
Analysis and/or data interpretation. |
|
JCD |
Final manuscript approval. |
REFERENCES
1. Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Estimativa 2018: incidência de câncer no Brasil / Instituto Nacional de Câncer José Alencar Gomes da Silva. Coordenação de Prevenção e Vigilância. Rio de Janeiro: INCA; 2017.
2. Malata CM, McIntosh SA, Purushotham AD. Immediate breast reconstruction after mastectomy for cancer. Br J Surg. 2000;87(11):1455-72. DOI: https://doi.org/10.1046/j.1365-2168.2000.01593.x
3. Hu E, Alderman AK. Breast reconstruction. Surg Clin North Am. 2007;87(2):453-67. DOI: https://doi.org/10.1016/j.suc.2007.01.004
4. Cosac OM, Costa LA, Barros APGSH. Reconstrução mamária bilateral com retalhos pediculados. In: Melega JM, Viterbo F, Mendes FH, eds. Cirurgia plástica: os princípios e a atualidade. Rio de Janeiro: Guanabara Koogan; 2011. p. 732-42.
5. Nahabedian MY. Factors to consider in breast reconstruction. Womens Health (Lond). 2015;11(3):325-42. DOI: https://doi.org/10.2217/WHE.14.85
6. Hartmann LC, Schaid DJ, Woods JE, Crotty TP, Myers JL, Arnold PG, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340(2):77-84. PMID: 9887158 DOI: https://doi.org/10.1056/NEJM199901143400201
7. Broët P, de la Rochefordière A, Scholl SM, Fourquet A, Mosseri V, Durand JC, et al. Contralateral breast cancer: annual incidence and risk parameters. J Clin Oncol. 1995;13(7):1578-83. PMID: 7602346 DOI: https://doi.org/10.1200/JCO.1995.13.7.1578
8. van Geel AN. Prophylactic mastectomy: the Rotterdam experience. Breast. 2003;12(6):357-61. DOI: https://doi.org/10.1016/S0960-9776(03)00136-X
9. Cosac OM, Ribeiro I, Moura LG, Soares DAS, Daher LMC, Galdino MCA, et al. Reconstrução mamária com loja de retalho retropeitoral superior e subcutâneo misto inferior. Rev Bras Cir Plást. 2018;33(2):166-73.
10. Tuttle TM, Jarosek S, Habermann EB, Arrington A, Abraham A, Morris TJ, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362-7. DOI: https://doi.org/10.1200/JCO.2008.20.1681
11. Stucky CC, Gray RJ, Wasif N, Dueck AC, Pockaj BA. Increase in contralateral prophylactic mastectomy: echoes of a bygone era? Surgical trends for unilateral breast cancer. Ann Surg Oncol. 2010;17(Suppl 3):330-7. DOI: https://doi.org/10.1245/s10434-010-1259-x
1. Hospital das Forças Armadas, Brasília, DF,
Brazil.
2. HOSPITAL DAHER LAGO SUL, BRASÍLIA, DF,
BRAZIL.
Corresponding author: Ognev Meireles Cosac, SMDB Conjunto 32, Lote 02, Casa A. Lago Sul, Brasilia, DF, Brazil. Zip Code: 71680-320. E-mail: ognev@terra.com.br
Article received: January 21, 2019.
Article accepted: February 10, 2019.
Conflicts of interest: none.








 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter