

Original Article - Year 2000 - Volume 15 -
Complications in Breast Augmentation
Complicações com Próteses Mamárias
ABSTRACT
The authors present their 25-year experience assisting patients in their private clinic and in their Educational Service. The increasing number of silicone prosthesis implants has increased the incidence of complications related to this procedure, but a systemic complication such as autoimmune or neoplastic disease was never observed. The most common local complications are studied and correlated to the different prostheses types manufactured over the years. The study of the prostheses coats and the organic fibrous capsules help to find the best decision in case of prosthesis replacement. These decisions may involve: peiform a capsulotomy or a capsulectomy, the best prosthesis coat type, the need of a documented information to the patient, the risks and benefits related to the procedure. Microscopic and macroscopic analysis of the local complications may help to decide the best prosthesis to be used: thin coat silicone gel prosthesis, textured lining coat prostheses, polyurethane overlapping coat prostheses, or inflatable saline-filled prostheses. There is no consensus about the ideal prosthesis. In conclusum, the squeezing maneuver (non-invasive manual compression of the hardened breast) must not be performed, thin coat siliconegel prostheses are more associated to complications and should not be used.
Keywords: Augmentation; mammaplasty and complications; silicone and complications; prosthesis and complications
RESUMO
Os autores apresentam sua experiência em 25 anos de atividade, em pacientes da clínica privada e do seu Serviço de Ensino, onde o número crescente de cirurgias de inclusão de próteses de silicone tem levado, conseqüentemente, à maior incidência de casos de complicações locais, sem que nunca se tenha detectado qualquer tipo de complicação de ordem sistêmica, tais como advento de doenças auto-imunes ou neoplásicas.
São analisadas as complicações locais mais comuns, correlacionando-as com os tipos de próteses utilizadas e seu período de fabricação. O estudo do envoltório das próteses, assim como das cápsulas orgânicas, deixam claro que se deve ponderar bastante, em caso de troca de próteses, quanto à capsulotomia ou capsulectomia, o tipo de revestimento da prótese, assim como a necessidade do esclarecimento documentado às pacientes, durante a primeira consulta, quanto às variantes favoráveis ou desfavoráveis inerentes a cada tipo de prótese.
A Documentação macro e microscópica das complicações locais são bastante elucidativas e visam contribuir para a decisão quanto ao tipo de prótese a se utilizar: gelatinosas lisas, gelatinosas texturizadas simples e com poliuretano, ou próteses texturizadas infláveis, salinas. Não existe consenso quanto à prótese ideal, mesmo porque ainda não podemos defini-la.
Conclui-se, definitivamente, que as manobras de squeeze (compressão manual externa, não invasiva, das mamas endurecidas) estão totalmente superadas, assim como as próteses de revestimento liso tendem a ser cada vez menos utilizadas, pela possibilidade de ocasionarem maiores complicações.
Palavras-chave: Mamaplastia e complicações; silicone e complicações; próteses e complicações
The number of silicone breast prostheses surgeries have significantly increased in the last 25 years. At the same time, the manufacture and the contents of the silicone breast prostheses have been modified.
Prostheses manufacturing development could be summarized as follows:
Breast augmentation surgeries usually achieve good results (Figs. 1a and 1b), but the following questions are frequently made when a patient decide to make a breast prosthesis implant:
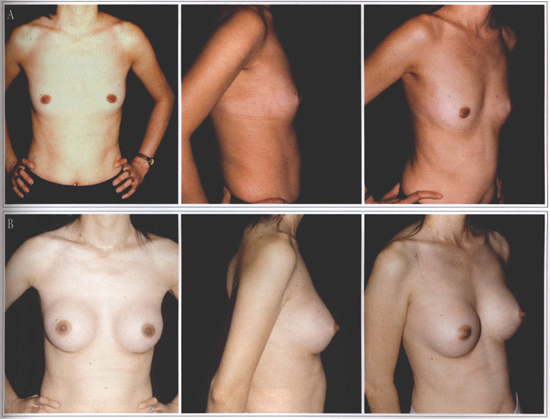
Fig. 1 - A: Hypomastia preoperative. B: Postoperative.
TECHNICAL DISCUSSION
The silicone (dimethyl-polysiloxane) is a very peculiar polymer: it is not biodegradable and is only dissolved in silicone itself. The silicone viscosity can be variable: from a very thin liquid to a gelatinous or solid consistency. The silicone viscosity is measured in centistokes. For example, silicone gel breast prostheses are constituted of a silicone coat having a higher centistoke (solid) than the inside gel.
The old thick coat prostheses with Dacron did not provide a natural esthetic result and the Dacron was also related to complications such as foreign body inflammatory reaction. In order to solve these problems, the prostheses manufacturers began to make thin coat prostheses. It created a different kind of complication: the silicone gel inside of the prosthesis would dissolve the thin coat and make it rupture over the time. When the covering of the thin coat prosthesis was ruptured or dissolved, the silicone gel inside the organic fibrous capsule could migrate to the contiguous tissues and cause: granuloma, skin inflammation with rash, urticaria, chronic pain, and calcifications. The extravasated silicone gel could also migrate to lymph nodes, along a peripheral nerve course, or into the chest. The silicone infiltrating the chest can simulate tumors and can require an exploratory thoracotomy (Fig. 2). There are also several cases of ruptured or dissolved coats that caused no symptoms. Abramo(1), Brandt(6), Brinton(7), Ferreira(10) and Shah(24) have studied the most common causes of periprosthetic breast capsules contraction since 1982.
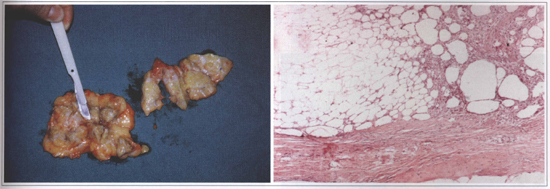
Fig.2 - Aspect of a 12 y. o. silicone implant. A: macroscopic view (siliconoma) of the breast. B: Microscopic view of the surrounding tissue infiltrated by silicone (silieone gel prosthesis rupture). Bilateral granulomas with exuberant fibrosis and foreign body rype reaction caused by silicone. Inflammatory process with lymphocytes. Breast parenchyma with ductal hyperplasia without atypia.
The thin coat prostheses should be improved, thus the manufacturers began to produce a double lumen prostheses (a silicone gel inside, an aqueous layer and a silicone coat). It was manufactured for a short time because it was not practical. At that time, the first inflatable prostheses were described(4,14) and manufactured(20,22,24) . Their valves were not very safe and the manufacturer recommended to fill those prostheses with macromolecular solurions (dextran). As in any type of prostheses, if the barrier between the prosthesis content and the body tissues is not effective, there will be exchanges between them, which can cause capsular contractions. In addition, the prosthesis could empty due to the poor quality valve.
Prostheses with a coat that would resist to the silicone gel were developed. They were called textured lining coat prostheses with a traditional silicone gel filling, a treated silicone gel (cohesive) filling, or other fillings such as castor oil and others. These last ones were not very well accepted by the physicians.
In order to protect the patient body, a product that would cover the silicone coat and minimize the capsular contraction should be developed. The polyurethane(18)showed to be the best product and the polyurethane overlapping coat prosthesis is still in use. Simultaneous to the polyurethane and textured coat prostheses development, the inflatable prostheses began to be studied again. The advent of tissue expanders helped to develop the inflatable prostheses. The safety of several types of valves was determined and an isotonic saline solution was recommended as the filling of these prostheses(20,22).
As in any other silicone prostheses, the inflatable prosthesis coat is not absolutely impermeable. A "bleeding" phenomenon (transudation from the prosthesis) may occur. Two causes are involved in this phenomenon: the pressure inside the prosthesis and the osmotic concentration of the liquid that fills it. If the pressure inside the prosthesis is hight, the filling liquid may transude and the prosthesis may deflate. The filling liquid also needs to be isotonic to avoid ions and water to exchange.
MATERIAL AND METHOD
Four hundred twenty primary or secondary patients with breast prostheses implants in the last 25 years were reviewed. The patients studied could present any type of complain or complication related to the prosthesis. The most common local complications observed were:
Fig. 3 - Baker grade VI capsular contraction.
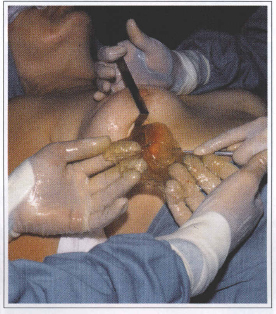
Fig. 4 - Ruptured silicone gel prosthesis. The prosthesis coat residues may be observed.
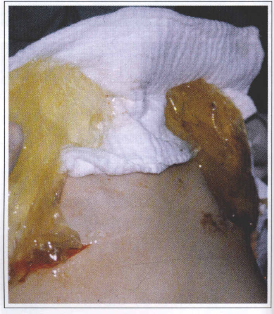
Fig. 5 - Total disappearance of the silicone prosthesis coat. The material corresponds to the filling gel.
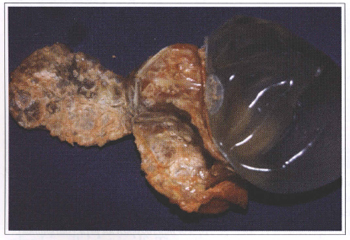
Fig. 6a - Organic fibrous capsule calcification around a silicone gel prosthesis placed 18 years ago.
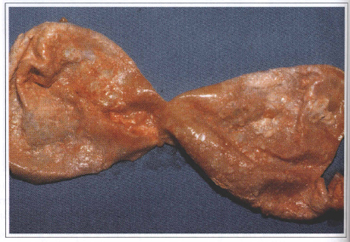
Fig. 6b - Another capsule with microscopic calcification.
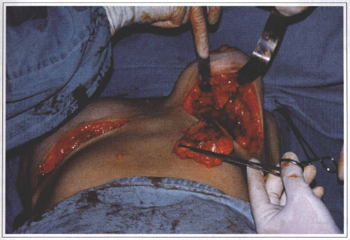
Fig. 7 - Silicone gel migration to the breast surrounding tissues. Note the large and hard tumors after a 15 years old prosthesis removal.
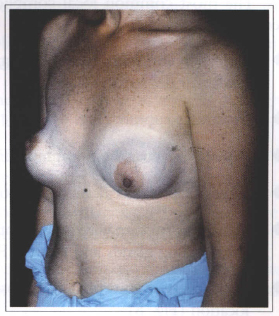
Fig. 8 - Postoperative dysmorphia and prosthesis dislocation.
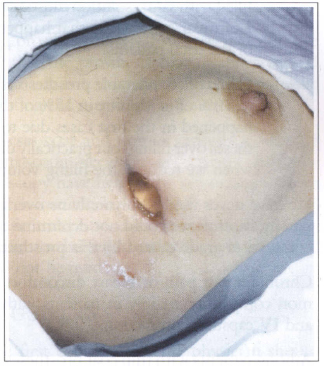
Fig. 9 - Prosthesis elimination due to infection.
In addition to the complications above mentioned, an extensive medical literature review revealed several other local complications such as: granulo mas formation, skin inflammation with rash, urticaria, and silicone gel migrating to lymph nodes, along a peripheral nerve course, and into the chest. The silicone gel migrating into the chest simulate tumors and may require exploratory thoracotomy(3,8,9,11).
Systemic complications (lupus erythematosus, seleroderma, rheumatism, and others) due to breast implant were never observed in our patients. In the literature, there is no data relating silicone breast implants to systemic complications. In our service, a prosthesis replacement due to its dislocation was performed in a patient with subcutaneous mastectomy and retromuscular breast implant. Fourty eight hours after the surgery there was an inflammatory reaction and the prosthesis was removed. This seemed to be a rejection type reaction, but immunocomplexes due to chemotherapy treatment were later identified as the cause. Six months later the prosthesis was implanted again and no complication was observed (Fig. 10).
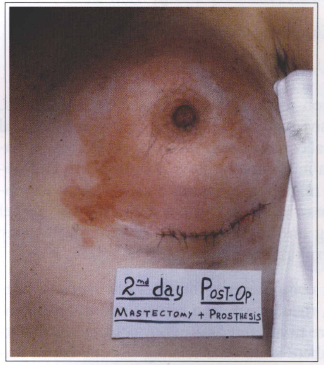
Fig. 10 - Inflammatory reaction 48 hours after the surgery. Rejection type reaction late characterized
as a immunocomplexes phenomena due to chemotherapy treatment.
Among the patients studied, the age varied between 26 and 50 years. In most patients the surgery was performed for esthetic reasons. There was also a significant number of patients submitted to a subcutaneous mastectomy and silicone breast implant that presented breast hardness due to the exiguous amount of tissue over the prosthesis. This was also observed in patients with retromuscular prostheses.
In patients with capsular contraction grades I to IV (Baker) and breast complaints, we observed that:
a) There was a predominance of grades III and IV in patients using a Phase 2 thin coat silicone gel prostheses (very thin coat prostheses).
b) Approximately 20% of the patients with severe contractions had the prosthesis coat ruptured or totally dissolved (Figs. 4 and 5).
c) Textured prostheses causes less contraction, usually II and III.
d) In the last seven years, inflatable prostheses filled with saline solution caused only a grade I capsular contraction that happened in 2% of the total patients.
e) Saline filied inflatable prosthesis emptied up to 10% of its volume in 15% of the cases. It happened in the first cases due to the prostheses over filling, but practically disappeared when we reduced the filling volume.
We have never used a polyurethane overlapping coat prosthesis, thus we could not determine the capsular contraction grade caused by this prosthesis type.
Chronic breast pain or breast discomfort were common complaints, mainly in patients with grades III and IV capsular contraction.
Among patients with thin coat prostheses, there have been a increasing number of patients with prosthesis rupture or prosthesis coat dissolution (Figs. 4 and 5). We began to systematically perform a capsulectomy in all patients undergoing prostheses replacement because the histological studies have shown that several capsules may have little amounts of silicone. In addition, silicone gel migration to the surrounded breast tissues create silicone tumors (siliconomas) (Figs. 2a and 2b).
The Food and Drugs Administration prohibited the silicone gel prosthesis use in the United States in 1992. Nowadays, only the inflatable saline filled prosthesis has been utilized. The silicone gel prostheses may be still used in the United States, in special cases, but this requires extensive documentation and approval. We have been using inflatable saline filled prostheses since 1993.
COMMENTS
During the last ten years, there have been an increasing number of breast implants and as a result, an increasing number of complications related to this procedure. These complications are most related to the kind of prosthesis utilized.
Some procedures had to be reviewed such as: whether the fibrous capsule around the implant should be left or not in case the prosthesis needs to be replaced and what type of prosthesis should be used for the replacement. The Baker's procedure was abandoned. It consisted of squeezing (non-invasive manual compression of the hardened breast). Several patients artributed the prosthesis coat rupture to that procedure and have sued their physician.
During old prosthesis removal surgery, it is not rare to observe that the prosthesis coat disappeared and silicone gel inside is restricted by the fibrous capsule or is infiltrating the surrounding tissues.
The prostheses coat and fibrous capsules studies by optical microscopy, electronic microscopy and scanning microscopy have helped the physicians and the patients to choose the best product to be used. Fibrous capsule and prosthesis surround tissues histological studies also helped to define the prosthesis type that should not be used.
CONCLUSIONS
During the first consultation, the physician should inform the patient the types of prostheses available and theirs advantages and disadvantages. He should also document the mutual option providing the patient an explanatory form and an authorizing form that must be signed. This will avoid a lawsuit in case of complications related to the prosthesis. If other complications occur, the informative document may attenuate the physician responsibility in case of a lawsuit.
However, the most important aspect of this documentation is to improve the relationship between the physician and the patient, thus eventual problems may be solved with mutual cooperation.
The complications related to the breast implants allow us to conclude:
1. The silicone coar prosthesis is semi-permeable.
2. The filling gel dissolves the prosthesis coar over the time.
3. Prostheses with textured coat are more resistant to capsular contraction.
4. The organic fibrous capsule is thicker when non texturized silicone gel prostheses are used.
5. The organic fibrous capsule is very thin if saline filled prostheses are used.
6. The capsular contraction is more severe with non texturized silicone gel prostheses.
7. The capsular contraction is less intense wich saline filled prostheses.
8. Inflatable saline prostheses can empty if over filled.
9. Patients should be clarified about me available prosthesis and theirs advantages and disadvantages.
10. The physician should give technical information to me patients and have the patient's consent signature before the procedure.
11. The squeeze procedures (non-invasive manual compression of hardened breast) must be avoided.
REFERENCES
1. ABRAMO AC. Proposition for prevention of capsular contractures in breast augmentation mammaplasty. An. Paul. Med. Cir. 1984;111(2,3):19-27.
2. ANDERSON RC, LARSON DL. Patient concerns related to media coverage of silicone implants. Plast. Surg. Nurs. 1995;15(2):89-91.
3. APESOS J, POPE TL Jr. Silicone granuloma following closed capsulotomy of mammary prosthesis. Ann. Plast. Surg. 1985;14(5):403-6.
4. BECKER H. Breast reconstruction using an inflatable breast implant with detachable reservoir. Plast. Reconstr. Surg. 1984;73(4):678-83.
5. BIGGS TM, CUKIER J, WORTHING LF. Augmentation mammaplasty: a review of 18 years. Plast. Reconstr. Surg. 1982;69(3):445-52.
6. BRANDT B, BREITING V, CHRISTENSEN L, NIELSEN M, THOMSEN JL. Five years experience of breast augmentation using silicone gel prostheses with emphasis on capsule shrinkage. Scand. J. Plast. Reconstr. Surg. 1984;18(3):311-6.
7. BRINTON LA, BROWN SL, COLTON T, BURICH MC, LUBIN J. Characteristics of a population of women with breast implants compared with women seeking other types of plastic surgery. Plast. Reconstr. Surg. 2000;105(3):919-29.
8. DUMBLE LJ. Dismissing the evidence: the medical response to women with silicone implant-related disorders. Health Care Women Int. 1996 Nov-Dec; 17(6):515-25.
9. EDWORTHY SM, MARTIN L, BARR SG, BIRDSELL DC, BRANT RF, FRITZLER MJ. A clinical study of the relationship between silicone breast implants and connective tissue. J. Rheumatol. 1998;25(2):254-60.
10. FERREIRA JA. The various etiological factors of "hard capsule" formation in breast augmentations. Aesth. Plast. Surg. 1984;8(2):109-17.
11. FOSTER WC, SPRINGFIELD DS, BROWN KL. Pseudotumor of the arm associated with rupture of silicone-gel breast prostheses. Report of two cases. J. Bone Joint Surg. Am. 1983;65(4):548-51.
12. GRUBER RP, FRIEDMAN GD. Periareolar subpectoral augmentation mammaplasty. Plast. Reconstr. Surg. 1981;67(4):453-7.
13. HAKME F. Contracted capsule. Complication of closed capsulotomy after silicone augmentation mammaplasty. Folha Méd. 1981;82(5):537-46.
14. JENNY H, SMAHEL J. Clinicopathologic correlations in pseudocapsule formation after breast augmentation. Aesth. Plast. Surg. 1981;5(1):63-8.
15. MAHLER D, HAUBEN DJ. Retromammary versus retropectoral breast augmentation: a comparative study. Ann. Plast. Surg. 1982;8(5):370-4.
16. NICOLAI JP. Consensus declaration on the safety of silicone breast implants. Plast. Reconstr. Surg. 1995;95(7):1339-40.
17. PARODI C. Implantes mamarios de siliconas. Legislación actual, requisitos y recomendaciones para el cirujano. Rev. Argent. Cir. Plast. 1996;2(2):146-7.
18. PENNISI VR. Polyurethane-covered silicone gel mammary prosthesis for successful breast reconstruction. Aesth. Plast. Surg. 1985;9(2):73-7.
19. PITANGUY I, ALEXANDRINO A, CALDEIRA AML, BRENTANO JMS, CARREIRÃO OS, CALIXTO CA. Inclusão de prótese subpeitoral pelo acesso transareolo-mamilar técnica de Pitanguy. Rev. Bras. Cir. 1984;74(3):149-62.
20. PRICE JE Jr. Capsular contraction and deflation associated with inflatable implants. Aesth. Plast. Surg. 1983;7(4):257-8.
21. PUCKETT CL. On the safety of silicone gel breast implants. Cancer Invest. 2000;18(3):278-80.
22. REIFFEL RS, REES TD, GUY CL, ASTON SJ. A comparison of capsule formation following breast augmentation by saline-filled or gel-filled implants. Aesth. Plast. Surg. 1983;7(2):113-6.
23. SARRABAYROUSE M. Implantes mamarios de gel de siliconas: solución o problema sobre las siliconas. Rev. Argent. Cir. Plást. 1997;3(2):75-7.
24. SHAH Z, LEHMAN JA Jr, STEVENSON G. Capsular contracture around silicone implants: the role of intraluminal antibiotics. Plast. Reconstr. Surg. 1982;69(5):809-14.
25. SHAH Z, LEHMAN JA Jr, TAN J. Does infection play a role in breast capsular contracture? Plast. Reconstr. Surg. 1981;68(1):34-42.
26. SPERLI A. Mastoplastias estéticas en las ptosis. El limite entre el uso de las siliconas y las mastopexias. Rev. Cir. Estética Argentina, 1977;2(2):93-8.
27. SPERLI A. Mammaplasty utilizing the Crossed Flap Technique, a critical analysis of 23 years experience. Rev. Soc. Bras. Cir. Plást. 1994;9(2,3) 34-46.
28. TERINO EO. Essential concepts in prosthetic breast surgery. Aesth. Plast Surg; 1982;6(1):25-32.
29. VANDERFORD ML, SMITH DH, OLIVE T. The image of plastic surgeons in news media coverage of the silicone breast implant controversy. Plast. Reconstr. Surg. 1995;96(3):521-38.
I - Professor of Surgery at Faculdade de Ciências Médicas da Santa Casa de São Paulo - Head of the Plastic Surgery Integrated Services, Hospital Ipiranga, São Paulo (SICP/HI) - SBCP-MEC.
II - Preceptors of the Plastic Surgery Integrated Services - SICP/HI SBCP-MEC.
III - Professor at the Surgical Pathology Department in Escola Paulista de Medicina.
Address for correspondence
Aymar Sperli, MD
Av.Cidade Jardim, 993
01453-000 - São Paulo - SP Brazil
Phone: (55 11) 3845-1279
e-mail: sperli@imedical.com
Study performed in the Plastic Surgery Integrated Services - SICP/HI - SBCP-MEC.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter