

Case Report - Year 2019 - Volume 34 -
Giant malignant fibrous histiocytoma of the face: case report of microsurgical repair using a transverse rectus abdominis myocutaneous flap
Fibrohistiocitoma maligno gigante de face: tratamento reparador microcirúrgico utilizando retalho miocutâneo transverso do reto do abdome - Relato de caso
ABSTRACT
Introduction: The resection of invasive tumors of the head and neck can result in extensive and complex defects requiring immediate repair. One repair option is the transfer of a transverse rectus abdominis myocutaneous (TRAM) flap pedicled on deep inferior epigastric vessels using vascular microsurgery. This study aimed to register a procedure used in the microsurgical treatment of giant malignant fibrous histiocytoma of the face using a TRAM flap.
Case Report: A male patient sought medical care for a giant tumoral lesion in the right hemiface. Computed tomography of the skull revealed a voluminous expansive process of vegetating aspect with poorly defined borders. The excision of the tumor affected the right masseter and temporalis muscles, parotid gland, and right orbital and malar bones. Subsequently, microsurgical withdrawal of the TRAM flap was performed with the deep inferior epigastric artery through a surgical incision in the hypogastric area. Dissection of the facial artery and vein under microscopy and venous and arterial anastomoses followed. The flap was intact with good perfusion and no signs of infection.
Conclusions: Microsurgical facial reconstruction allows head and neck surgeons to resect large tumors.
Keywords: Myocutaneous flap; Reconstructive surgical procedures; Neoplasms; Rectus abdominis; Face
RESUMO
Introdução: A ressecção de tumores invasivos de cabeça e pescoço pode resultar em defeitos extensos e complexos exigindo reparação imediata. Uma das opções de reparação é a transferência, utilizando técnica de microcirurgia vascular, do retalho musculocutâneo do reto abdominal pediculado nos vasos epigástricos inferiores profundos (TRAM). O presente estudo tem como objetivo registrar um procedimento utilizado no tratamento reparador microcirúrgico de fibrohistiocitoma maligno gigante de face com retalho TRAM.
Relato de Caso: Paciente procurou atendimento médico devido a lesão tumoral gigante em hemiface direita. Foi realizada a tomografia computadorizada do crânio revelando volumoso processo expansivo de aspecto vegetante com limites mal definidos. Após os procedimentos básicos no pré-operatório, realizou-se a exérese do tumor que acometia músculos masseter e temporal direito, glândula parótida, assoalho orbitário à direita e osso malar. Posteriormente, retirou-se o retalho microcirúrgico do músculo reto do abdome em conjunto com a artéria epigástrica inferior profunda através de incisão cirúrgica da área hipogástrica. Em seguida, dissecção da artéria e veia facial utilizando microscópio e anastomoses venosa e arterial. Quanto à evolução retalho apresentou-se íntegro, com boa perfusão, sem sinais de infecção.
Conclusões: A reconstrução facial microcirúrgica oferece liberdade ao cirurgião de cabeça e pescoço para realizar grandes ressecções tumorais.
Palavras-chave: Retalho miocutâneo; Procedimentos cirúrgicos reconstrutivos; Neoplasias; Reto do abdome; Face
INTRODUCTION
Malignant fibrous histiocytoma is a malignant mesenchymal neoplasm (sarcoma) of soft tissues in which histiocytes act as facultative fibroblasts or some elements of the primitive mesenchyme give rise to fibroblasts and histiocytes1-4; malignant fibrous histiocytoma can occur anywhere in the body. Due to tumor aggression, complete and early resection of the lesion with free margins accompanied by regional lymph node excision is the therapeutic approach indicated in all cases of malignant fibrous histiocytoma2,5.
The resection of large invasive tumors of the head and neck can result in extensive and complex defects, leading to the exposure of vital structures as well as direct communication between the oronasopharynx and the brain, thus requiring immediate repair. These patients may have significant limitations, with high morbidity rates and decreased quality of life6,7.
Accordingly, several microsurgical flaps have been used to repair defects of the head and neck region1. Several studies have asserted the superiority of free musculocutaneous flaps over fasciocutaneous flaps, the most common being the rectus abdominis flap, although the anterolateral free thigh flap is also widely used6,8-11.
The advantages of using the rectus abdominis flap include its low incidence of complications, the ease of its elevation, and the presence of a long, large-caliber, and constant vascular pedicle represented by the deep inferior epigastric artery8,11.
Thus, the objective of this case report is to present the microsurgical repair of a patient with a malignant giant fibrous histiocytoma of the face using a transverse rectus abdominis myocutaneous (TRAM) flap.
CASE REPORT
This study was performed in accordance with the precepts of the Declaration of Helsinki and the Nuremberg Code respecting the Research Regulations Involving Human Beings (Resolution CNS 196/96) of the National Health Council. This retrospective study used data obtained through semi-structured interviews, direct observations, and documentary assessments that included the patient’s medical records; these steps were performed after approval of the draft project by the Research and Extension Nucleus of Medicine and Ethics Commission of the State University of Pará and authorized by the Clinical Director of the Ophir Loyola Hospital and the patient through an informed consent form.
History
A 56-year-old man sought medical care for a giant tumor lesion in the right hemiface. He reported that it first developed in 1995 as an erythematous papule in the right malar region and progressively grew to an ulcerative-vegetative lesion on the face. The patient sought medical assistance with the initial diagnosis of American cutaneous leishmaniasis and treatment with N-methyl glucamine; without improvement, a biopsy revealed squamous cell carcinoma, for which he was referred to our service for radiotherapy (RT).
Two years after the initial treatment, a new lesion emerged in the right hemiface accompanied by local burning pain for which new RT sessions were instituted.
Five years later, in 2002, a fast-growing ulcerative mass appeared on the scar lesion produced by RT and was accompanied by local moderate-intensity pain and the secretion of a foul-smelling purulent bloody fluid.
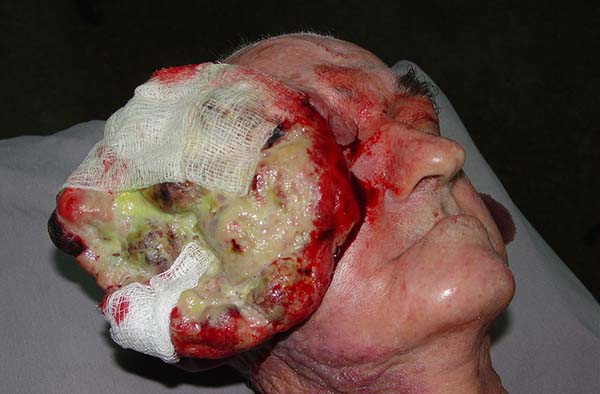
On performing a physical examination, it was found that the patient was emaciated with a hyperemic ulcerative-vegetative lesion with a giant necrotic and infected center in the right hemiface that extended to the ipsilateral orbit measuring 11 × 10 cm and with inflammatory signs (Figure 1). The patient’s right hand was missing as a result of a work accident. Examinations of the thorax and abdomen were unaltered, and a hemogram revealed hypochromic and microcytic anemia and leukocytosis.
Computed tomography of the skull revealed a large expansive process with a vegetating aspect and poorly defined borders compromising soft parts; signs of bone destruction of the walls of the zygomatic arch; impairment of the right temporal muscle; and an intimate relationship with the right eyelid region and the anterior edge of the eyeball.
In 2003, the tumor was resected, followed by microsurgical reconstruction with a TRAM flap.
Surgical technique
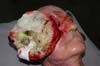
The procedure began with a perilesional incision and careful dissection of the tumor lesion, followed by excision of the tumor that involved the masseter and right temporal muscles, parotid gland, orbital floor dissection to the right, and malar bone and submandibular lymph node dissection to the right.
Subsequently, a surgical incision was made in the hypogastric area, including the entire infraumbilical area, which was 21 × 37 cm in its major dimensions, and the hypogastric segment was detached accompanied by dissection of the deep inferior epigastric artery (IEA) and ligation of the vein and the IEA with removal of the microsurgical flap.
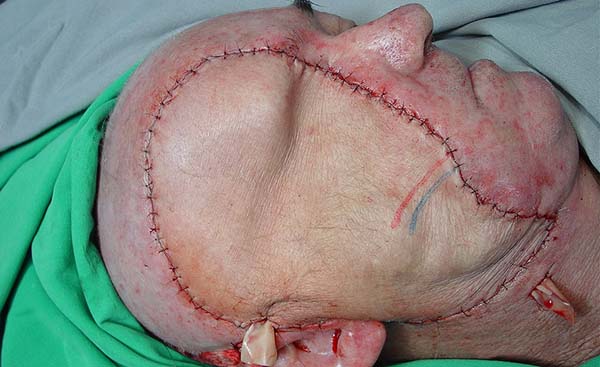
Dissection of the facial artery and vein was done using a 40× magnification microscope, and venous and arterial end-to-end anastomoses of the facial vein and artery with IEA vessels using 10-0 mononylon wire were performed. Patency and flow success were verified using appropriate microsurgical instruments, followed by fixation of the TRAM flap in the resected area using Vicryl 2-0 wire (Figure 2).
RESULTS
After the surgical procedure, the patient was transferred to the Intensive Therapy Center, where he remained for 2 days and was medicated with dobutamine and dopamine for hypotension. The flap was viable with good perfusion and no signs of ischemia; antibiotic therapy was continued.
On the fifth postoperative day, a purulent secretion was noted in the drain. A new antibiotic regimen was initiated; after some adjustments due to diarrheal episodes, it was maintained until discharge 16 days after surgery when the patient was in a good general condition with an intact TRAM flap.
A histopathological examination of the collected material showed poorly differentiated epidermoid carcinoma, Broders grade III. An immunohistochemical evaluation with HMB-45, S-100, vimentin, PCNA, AE1, and AE2 antigens and cytokeratin showed that the lesion was of mesenchymal origin and was compatible with malignant fibrous histiocytoma with high proliferative activity.
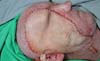
Eight months after surgery, the patient returned to the outpatient clinic with an intact flap with good perfusion and no signs of infection or increased volume. The suture line was in a good scarring condition. He reported a difference in the skin coloration of his face and the flap. Deviation of the labial commissure to the left was evident, as was weakness in the abdominal wall in the flap donor area.
DISCUSSION
Here we opted to use a TRAM flap to correct facial defects after tumor excision for its functional and esthetic advantages9, absence of previous abdominal surgeries in the patient, technical ease of the flap dissection by a qualified professional, previous tumor resection not requiring a change in decubitus, and the flap’s versatility.
Studies have indicated that necrosis of the transferred flap is the most common complication of this microsurgical repair technique6,12; previous RT is a risk factor due to its effects on recipient vessels. This causes greater difficulty with vessel dissection and the preparation for vascular anastomosis6,13.
However, in this study, no necrosis of the TRAM flap was observed despite a history of RT. Another complication is incisional hernia in the donor area, which can easily be circumvented with the use of an absorbable suture without reinforcement and synthetic material mesh6,8,10 in the follow-up period (12 months). In this case, fragility of the abdominal wall (donor area) was observed after flap withdrawal as described elsewhere in the literature.
The difference in color between the skin of the face and the flap as reported in the literature was quite visible in this patient in the initial phase, but it decreased gradually over time6.
Regarding the clinical manifestation of malignant fibrous histiocytoma, this case was uncommon since such tumors in the head and neck region are rare, the condition is more common in children, and the tumor is usually 1-2 cm in diameter according to the literature surveyed1-4. This case involved an 11-cm tumor in a sexagenarian.
CONCLUSIONS
Microsurgical facial reconstruction, especially using a TRAM flap, enables the head and neck surgeon to perform large tumor resections and preserve the quality of life of cancer patients.
ACKNOWLEDGMENTS
The authors thank Dr. Maria Vanda C. Arnaud for performing immunohistochemistry analysis, Dr. Tathiane Lamarão Vieira De Graaf for providing slides for the histopathological diagnosis, and Dr. Sâmia Demachki for imaging the histopathology slides.
We also thank our friends Kallene Summer Vidal, Lorena Vidal, Rodrigo Cordovil, Ana Júlia, Alfredo Nadir Abud Neto, and Karen Roberta N. Souza for their help with this study.
COLLABORATIONS
|
RAAA |
Analysis and/or data interpretation, final manuscript approval, supervision, writing - review & editing. |
|
BRAP |
Contribution: supervision, writing - review & editing. |
|
RCCA |
Analysis and/or data interpretation, conception and design study, data curation, writing - original draft preparation, writing - review & editing. |
|
BFAP |
Conception and design study, data curation. |
REFERENCES
1. Ichikawa E, Furuta J, Mochizuki T, Imakado S, Otsuka F. Cutaneous malignant fibrous histiocytoma of the face. Int J Dermatol. 2003;42(12):952-4. DOI: http://dx.doi.org/10.1111/j.1365-4632.2003.01839.x
2. Rothman AE, Lowitt MH, Pfau RG. Pediatric cutaneous malignant fibrous histiocytoma. J Am Acad Dermatol. 2000;42(2 Pt 2):371-3.
3. Stadler FJ, Scott GA, Brown MD. Malignant fibrous tumors. Semin Cutan Med Surg. 1998;17(2):141-52. DOI: http://dx.doi.org/10.1016/S1085-5629(98)80007-2
4. Coffin CM, Dehner LP, O'shea PA. Pediatric soft tissue tumors: a clinical, pathological and therapeutic approach. Baltimore: Williams and Wilkins; 1997.
5. Yip D, Stacy GS. Malignant fibrous histiocytoma soft tissue. On line. Chicago. Atualizado em 29 de Agosto de 2002. Acessado 2004 Abr 3.
6. Aki FE, Besteiro JM, Ferreira MC. Reparação Imediata de Defeitos Complexos de Cabeça e Pescoço com o Retalho Microcirúrgico Músculo-Cutâneo do Reto Abdominal. Rev Bras Cir Plást. 1997;12(3):37-54.
7. Gao RW, Nuyen BA, Divi V, Sirjani D, Rosenthal EL. Outcomes in Head and Neck Resections That Require Multiple-Flap Reconstructions: A Systematic Review. JAMA Otolaryngol Head Neck Surg. 2018;144(8):746-52. DOI: http://dx.doi.org/10.1001/jamaoto.2018.0835
8. Meland NB, Fisher J, Irons GB, Wood MB, Cooney WP. Experience with 80 rectus abdominis free-tissue transfers. Plast Reconstr Surg. 1989;83(3):481-7. DOI: http://dx.doi.org/10.1097/00006534-198903000-00014
9. Nakatsuka T, Harii K, Yamada A, Asato H, Ebihara S. Versatility of a free inferior rectus abdominis flap for head and neck reconstruction: analysis of 200 cases. Plast Reconstr Surg. 1994;93(4):762-9. DOI: http://dx.doi.org/10.1097/00006534-199404000-00017
10. Sofiadellis F, Liu DS, Webb A, Macgill K, Rozen WM, Ashton MW. Fasciocutaneous free flaps are more reliable than muscle free flaps in lower limb trauma reconstruction: experience in a single trauma center. J Reconstr Microsurg. 2012;28(5):333-40. DOI: http://dx.doi.org/10.1055/s-0032-1313764
11. Zuker RM, Manktelow RT, Palmer JA, Rosen IB. Head and neck reconstruction following resection of carcinoma, using microvascular free flaps. Surgery. 1980;88(4):461-6.
12. Vargo JD, Przylecki W, Camarata PJ, Andrews BT. Classification and Microvascular Flap Selection for Anterior Cranial Fossa Reconstruction. J Reconstr Microsurg. 2018;34(8):590-600. DOI: http://dx.doi.org/10.1055/s-0038-1649520
13. Achauer BM, Salibian AH, Furnas DW. Free flaps to the head and neck. Head Neck Surg. 1982;4(4):315-23. DOI: http://dx.doi.org/10.1002/hed.2890040409
1. Hospital Ophir Loyola, Belém, PA,
Brazil
2. Universidade Estadual do Pará, Belém, PA,
Brazil
3. Universidade Federal do Pará, Belém, PA,
Brazil.
Corresponding author: Rui Antonio Aquino de Azevedo Padre Eutíquio, nº 1380 - Batista Campos, Belém, PA, Brazil Zip Code 66035-045 E-mail: drruiazevedo@hotmail.com
Article received: March 8, 2018.
Article accepted: November 11, 2018.
Conflicts of interest: none.


 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter