

Original Article - Year 2019 - Volume 34 -
Prospective assessment of the repercussions on the lipid profile of surgeries involving liposuction and dermolipectomies
Avaliação prospectiva das repercussões no perfil lipídico das cirurgias que envolvem lipoaspiração e dermolipectomias
ABSTRACT
Introduction: Liposuction associated with dermolipectomies is the most commonly performed surgical procedure in plastic surgery. Although regarded as an extremely safe surgery, some considerations must be taken on the possible metabolic effects of these surgeries. The development of the tumescent technique in liposuction allowed the safer removal of large amounts of fat. The objective is to compare lipid profile variations in the early and late postoperative period in patients undergoing liposuction and dermolipectomies.
Methods: Between October 2006 and June 2012, 40 female patients who were candidates for surgeries involving liposuction and dermolipectomies were prospectively followed, and the lipid profile was analyzed through preoperative and postoperative examinations. The surgeries performed were mammoplasty + liposuction, abdominoplasty + liposuction, and lipoabdominoplasty + mammoplasty.
Results: Of the 40 female patients who were followed, 20 were selected (after applying the exclusion criteria). In agreement with our study, in 1996, Cazes showed that there were no changes in the lipid profile of patients 12 months after lipoabdominoplasty.
Conclusion: After a preoperative and postoperative analysis of 20 patients, it was observed that there were no statistically significant changes in the lipid profile and that the measurements after 1 year were close to those obtained in the preoperative period.
Keywords: Lipid metabolism disorders; Lipid A; Abdominoplasty; Lipectomy; Triglycerides
RESUMO
Introdução: Lipoaspiração associada a dermolipectomias é o procedimento cirúrgico mais comumente realizado em cirurgia plástica. Apesar de ser considerada uma cirurgia extremamente segura, algumas considerações devem ser levantadas a respeito dos possíveis efeitos metabólicos que essas cirurgias possam causar. O desenvolvimento da técnica tumescente de lipoaspiração permitiu a remoção de grande quantidade de gordura de modo mais seguro. O objetivo é comparar as variações do perfil lipídico em pós-operatório precoce e tardio de pacientes submetidos à lipoaspiração e dermolipectomias.
Métodos: Entre outubro de 2006 e junho de 2012, 40 pacientes do sexo feminino candidatas a cirurgias que envolviam lipoaspiração e dermolipectomias foram acompanhadas prospectivamente e o perfil lipídico foi analisado por meio de exames no pré-operatório e no pós-operatório. As cirurgias realizadas foram: mamoplastia + lipoaspiração, abdominoplastia + lipoaspiração e lipoabdominoplastia + mamoplastia.
Resultados: Das 40 pacientes que foram acompanhadas no estudo, 20 pacientes do sexo feminino foram selecionadas (após a aplicação dos critérios de exclusão). Em consonância com nosso estudo, Cazes, em 1996, demonstrou que após 12 meses de pós-operatório de lipoabdominoplastia não houve alteração do perfil lipídico das pacientes.
Conclusão: Após análise pré- e pós-operatória de 20 pacientes, observamos que não há alterações estatísticas significantes em relação ao perfil lipídico com tendência de equilíbrio das aferições em um ano em patamares próximos aos observados no pré-operatório.
Palavras-chave: Transtornos do metabolismo dos lipídeos; Lipídeo A; Abdominoplastia; Lipectomia; Triglicerídeos
INTRODUCTION
In plastic surgery, body-contouring surgery is currently becoming increasingly popular due to the appreciation of a well-toned body in modern times. Liposuction associated with lipectomy is one of the most performed surgical procedures in plastic surgery. Although regarded as a safe surgery, considerations should be taken on its possible metabolic effects1.
It is presently known that the subcutaneous tissue acts as an endocrine organ that produces adipocytokines that help maintain homeostasis. Based on this, some plastic surgeons have assessed the metabolic effects of liposuction on fat reduction. Another associated procedure that removes fat from the subcutaneous tissue is dermolipectomy2.
The development of the tumescent technique for liposuction allowed a safer removal of large amounts of fat. With the knowledge that the adipose tissue is an endocrine organ, this alternative led researchers to believe that liposuction could be a viable method for improving the metabolic profile through immediate loss of body fat mass, a possible coadjuvant in the treatment of obesity and comorbidities3, associated with physical activity and lifestyle changes.
Studies in humans suggest that large-volume liposuction can increase the proportion of visceral adipose tissue compared to abdominal tissue, and this leads us to think of possible metabolic complications related to the procedure4.
Does liposuction have repercussions on the lipid profile of patients undergoing body-contouring surgery? In medical literature, there are several studies that show dissonant and sometimes contradictory conclusions regarding alterations in the lipid profile of patients undergoing liposuction and dermolipectomies3,4.
OBJECTIVE
The objective of this study was to compare the lipid profile variations in the early and late postoperative period in patients undergoing liposuction and dermolipectomy.
METHODS
Between October 2006 and June 2012, the lipid profile of 40 female patients undergoing liposuction and dermolipectomy was analyzed through preoperative and postoperative examinations.
The surgeries performed were mammoplasty + liposuction, abdominoplasty + liposuction, and lipoabdominoplasty + mammoplasty. The patients were divided into 2 groups:
A comparative analysis of total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides was performed for the groups. Three comparisons were performed: In group 1, the results of the preoperative examinations were compared to the 3-month postoperative results. In group 2, the results of the preoperative examinations were compared to the 1-year postoperative results. After this analysis, the 3-month postoperative results of group 1 were compared to the 1-year postoperative results of group 2.
All patients in the study were aware of the need to return periodically to the clinic in the postoperative period to undergo blood tests and clinical assessments.
The inclusion criteria were as follows: participation and willingness to undergo complementary examinations relevant for the study in predetermined times; volume of liposuction not exceeding 5–7% of the body weight as recommended by Resolution No. 1711 of December 10, 2003, of the Brazilian Federal Council of Medicine (Conselho Federal de Medicina), use of the tumescent technique5 and surgical risk ASA I or II. The exclusion criteria were as follows: body mass index (BMI) > 30 kg/m2 before dietary re-education and weight reduction, associated with physical activity; gastroplasty; surgeries combined with another specialty; gigantomastia (estimated weight of the resection > 800–1000 g) associated with abdominal apron (estimated weight of resections > 2000 g) and lipodystrophy of the flanks and dorsum (large volume and area liposuctions were estimated), except if patients agree to undergo only lipoabdominoplasty and liposuction of the flanks in the first procedure and mammoplasty in the future; changes in their physical condition in the 1-year postoperative period or increase of ≥ 2 kg/m2 in the BMI; pregnancy after surgery; and dyslipidemia with medication use for such a pathology.
Operations in all patients were performed by a single plastic surgeon using the same surgical technique and from private practice. The laboratory tests were performed in the pre- and postoperative period in the same laboratory.
Statistical tests were performed with significance level set at a P-value < 0.05. All patients were advised about the postoperative period and importance of performing physical activity pre- and postoperatively.
RESULTS
Of the 40 patients who were followed in the study, 20 were selected (after applying the exclusion criteria).
Of the 20 excluded patients, 7 showed changes in the BMI; 6 patients were not present in the follow-up consultation and did not undergo postoperative examinations; 5 showed changes in physical condition; and 2 patients were excluded as they had dyslipidemia and were using statin.
The analyzed patients were aged between 30 and 59 years and had BMI < 30 kg/m2, with an average of 26.4 kg/m2 (SD = 2.2). The mean liposuction volume was 3,415 mL (SD = 1.024). The patients had a mean total cholesterol level of 197.7 mg/dL (SD = 34.3), LDL level of 118.4 mg/dL (SD = 24.6), HDL level of 51.3 mg/dL (SD = 10.7), and triglyceride level of 127.2 mg/dL (SD = 60.8). All patients underwent lipoabdominoplasty and liposculpture surgeries, and 7 (35%) also underwent breast surgery (Table 1).
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Age | 43 | 9 | 30 | 59 |
| BMI | 26.4 | 2.2 | 22.3 | 30.0 |
| Liposuction volume | 3,415 | 1,024 | 1,050 | 4,800 |
| Total cholesterol | 197.7 | 34.3 | 124.0 | 260.0 |
| LDL | 118.4 | 24.6 | 80.0 | 174.2 |
| HDL | 51.3 | 10.7 | 36.0 | 73.0 |
| TRIG | 127.2 | 60.8 | 39.0 | 277.0 |
| Surgeries Performed | N | % | ||
| Lipoabdominoplasty and liposuction | 7 | 35% | ||
| Lipoabdominoplasty, liposuction, and mammoplasty | 13 | 65% |
Table 2 shows data on the profile of selected patients and preoperative data in each group. Although there are no preoperative parameters that distinguish groups 1 and 2, this analysis had to evaluate the homogeneity between groups to subsequently compare the 3-month and 1-year postoperative measurements.
| Group 1 | Group 2 | Mann-Whitney U
test (P-value) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | ||
| Age | 43 | 10 | 30 | 59 | 43 | 10 | 32 | 57 | 0.9710 |
| BMI | 26.7 | 2.2 | 26.0 | 2.3 | 0.3930 | ||||
| Liposuction volume | 3,555 | 993 | 1,050 | 4,800 | 3,275 | 1,088 | 1,400 | 4,600 | 0.4810 |
| Total cholesterol | 195.7 | 42.0 | 160.0 | 241.0 | 199.7 | 26.6 | 124.0 | 260.0 | 0.6840 |
| LDL | 118.0 | 31.5 | 97.0 | 148.0 | 118.7 | 16.9 | 80.0 | 174.2 | 1.0000 |
| HDL | 50.8 | 10.2 | 37.0 | 73.0 | 51.8 | 11.7 | 36.0 | 64.0 | 1.0000 |
| TRIG | 112.6 | 61.2 | 66.0 | 277.0 | 141.7 | 60.0 | 39.0 | 221.0 | 0.2180 |
As it can be observed, the groups did not show statistically significant differences (p > 0.05) regarding the analyzed variables (age, BMI, liposuction volume, and preoperative total cholesterol, LDL, HDL, and triglyceride levels) and can therefore be considered a single sample.
The subsequent analyses compared the pre- and postoperative lipid measurements, and group 1 was assessed 3 months postoperatively and group 2 was assessed 1 year postoperatively.
The analysis in group 1 showed a decrease in the mean total cholesterol and LDL levels and an increase in HDL and triglyceride levels. However, no significant differences (p > 0.05) were found between the preoperative and 3-month postoperative measurements (Table 3).
| Preoperative | Postoperative (3 months) |
Wilcoxon signed rank
test (P-value) |
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Total cholesterol | 195.70 | 42.02 | 192.80 | 23.87 | 0.799 |
| LDL | 118.04 | 31.53 | 117.18 | 21.00 | 0.721 |
| HDL | 50.80 | 10.17 | 53.54 | 7.67 | 0.333 |
| TRIG | 112.60 | 61.19 | 113.40 | 40.24 | 0.646 |
Contrary to group 1, the analysis in group 2 showed an increase in the mean total cholesterol and LDL levels and a decrease in HDL and triglyceride levels. However, no significant differences (p > 0.05) were found between the preoperative and 1-year postoperative measurements (Table 4).
| Preoperative | Postoperative (1 year) |
Wilcoxon signed rank
test (P-value) |
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Total cholesterol | 199.70 | 26.61 | 202.70 | 20.69 | 0.721 |
| LDL | 118.70 | 16.87 | 122.00 | 34.02 | 0.314 |
| HDL | 51.80 | 11.66 | 47.90 | 7.08 | 0.138 |
| TRIG | 141.70 | 59.96 | 135.60 | 33.96 | 0.760 |
The subsequent analysis compared the 3-month postoperative measurements of group 1 with the 1-year postoperative measurements of group 2. This analysis was possible as the groups showed homogeneous preoperative measurements. However, because the measurements do not belong to the same group of patients in the two analyzed periods, the Mann-Whitney U test was used to determine whether the postoperative cholesterol measurements differ between the groups (Table 5).
| Postoperative (3 months) |
Postoperative (1 year) |
Wilcoxon signed rank
test (P-value) |
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Total cholesterol | 192.80 | 23.87 | 202.70 | 20.69 | 0.9120 |
| LDL | 117.18 | 21.00 | 122.00 | 34.02 | 0.6840 |
| HDL | 53.54 | 7.67 | 47.90 | 7.08 | 0.1900 |
| TRIG | 113.40 | 40.24 | 135.60 | 33.96 | 0.2180 |
The measurements showed no statistically significant differences, but there was a trend in the increase in total cholesterol and LDL levels and decrease in HDL and triglyceride levels.
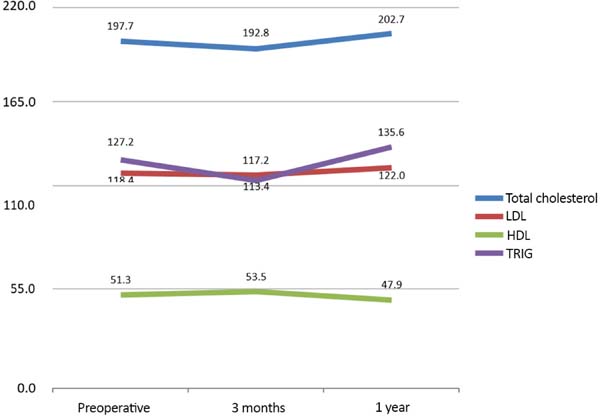
Figure 1 shows the mean cholesterol measurements at the three analyzed periods. Although the comparison between the periods was not statistically conclusive, there is a trend for alterations in the 3-month postoperative measurements and equilibrium in the 1-year postoperative measurements with values close to those observed in the preoperative period.
The next analysis is aimed at identifying possible correlations between liposuction volume and cholesterol measurements.
Correlation analysis was conducted to measure the degree of association between two variables. Spearman’s coefficient was used in this analysis.
A significance test was then performed with the initial hypothesis that there is no association between the variables. P-values < 0.05 indicate a significant association (Table 6).
| Correlation | G1, postoperative (3 months) | G2, postoperative (1 year) | ||
|---|---|---|---|---|
| Spearman's coefficient | P-value | Spearman's coefficient | P-value | |
| Liposuction volume × total cholesterol | 0.11 | 0.3814 | 0.05 | 0.4433 |
| Liposuction volume × LDL | 0.17 | 0.3186 | -0.21 | 0.2769 |
| Liposuction volume × HDL | -0.34 | 0.1717 | -0.34 | 0.1710 |
| Liposuction volume × TRIG | 0.48 | 0.0793 | 0.03 | 0.4667 |
The following correlations indicate that the difference between the preoperative and 3-month postoperative triglyceride levels has a direct association with liposuction volume, although inconclusive (p = 0.0793). Conversely, there was no trend in the 1-year postoperative values (p = 0.4667). Thus, considering the 3-month postoperative period, higher liposuction volumes lead to greater decreases in triglyceride levels. Moreover, 1 year postoperatively, the liposuction volume no longer influences the triglyceride levels.
In other cholesterol measurements, no statistically significant correlations were observed. However, for HDL, both the 3-month and 1-year coefficients were negative, indicating that there is a trend, although inconclusive, and that higher liposuction volumes lead to less pronounced decreases in HDL levels.
DISCUSSION
Removal of a significant volume of fat from the subcutaneous tissue through liposuction creates a visible change in body composition through a rapid decline in subcutaneous adipose tissue. There are beneficial effects on traditional forms of weight loss, in which both subcutaneous and intra-abdominal adipose tissues are reduced. However, physiological and metabolic effects that result only from subcutaneous fat loss are still not well established.
The subcutaneous fat has different metabolic implications compared to visceral adipose tissue and is the main source of energy and free fatty acids.
Although experimental animal studies have shown that fat elimination from the body improves serum lipid levels, some studies in humans suggested that large-volume liposuction can increase the proportion of visceral adipose tissue, which raises the concern of possible metabolic complications related to liposuction and increases the risk of cardiovascular diseases4. Matarasso et al.5 assessed the impact of liposuction on body fat and concluded that although large-volume liposuction removes a small amount of fat from the body, this significantly increases the proportion of visceral fat.
Samdal et al.6 assessed patients undergoing large-volume liposuction and their lipid profiles in the preoperative and postoperative periods (1, 9, and 12 months after surgery). The major finding of the study was a significant increase in HDL level in all patients. Based on the mean HDL level increase of 0.2 mm/L, the authors showed that large-volume liposuction can reduce the risk of cardiovascular disease by up to 30%. In our case series, there was no improvement in HDL levels 1 year postoperatively.
Capla and Rubin7 prospectively analyzed 322 patients who underwent liposuction and/or abdominoplasty and the impact on the lipid profile after 3 months. The study showed a significant reduction in triglyceride levels and no changes in cholesterol levels. In our study, it was observed that at 3 months postoperatively, LDL and total cholesterol levels improved and triglyceride levels worsened, but these changes were not statistically significant.
In agreement with our study, Cazes, in 1996, showed that there were no changes in the lipid profile of patients undergoing lipoabdominoplasty 12 months postoperatively. Associated with this, the study also showed the efficacy of physical exercise on the lipid profile of patients, which reduces the lipogram after the metabolic stress caused by liposuction8,9.
CONCLUSION
After the preoperative and postoperative analyses of 20 patients undergoing liposuction and dermolipectomies, no statistically significant changes were observed in the lipid profile, with a trend to equilibrium 1 year postoperatively to levels close to that observed in the preoperative period.
COLLABORATIONS
|
LDPB |
Analysis and/or data interpretation, conception and design study, conceptualization, data curation, final manuscript approval, formal analysis, funding acquisition, investigation, methodology, project administration, realization of operations and/or trials, resources, software, supervision, validation, visualization, writing - original draft preparation, writing - review & editing. |
|
JDLGA |
Analysis and/or data interpretation, conception and design study, conceptualization, data curation, final manuscript approval, formal analysis, funding acquisition, investigation, methodology, project administration, realization of operations and/or trials, resources, software, supervision, validation, visualization, writing - original draft preparation, writing - review & editing. |
|
MB |
Analysis and/or data interpretation, conception and design study, conceptualization, data curation, final manuscript approval, formal analysis, funding acquisition, investigation, methodology, project administration, realization of operations and/or trials, resources, software, supervision, validation, visualization, writing - original draft preparation, writing - review & editing. |
|
GCS |
Analysis and/or data interpretation, conception and design study, conceptualization, data curation, final manuscript approval, formal analysis, funding acquisition, investigation, methodology, project administration, realization of operations and/or trials, resources, software, supervision, validation, visualization, writing - original draft preparation, writing - review & editing. |
|
ACC |
Analysis and/or data interpretation, conception and design study, conceptualization, data curation, final manuscript approval, formal analysis, funding acquisition, investigation, methodology, project administration, realization of operations and/or trials, resources, software, supervision, validation, visualization, writing - original draft preparation, writing - review & editing. |
|
RCSD |
Analysis and/or data interpretation, conception and design study, conceptualization, data curation, final manuscript approval, formal analysis, funding acquisition, investigation, methodology, project administration, realization of operations and/or trials, resources, software, supervision, validation, visualization, writing - original draft preparation, writing - review & editing. |
|
JGOJ |
Analysis and/or data interpretation, conception and design study, conceptualization, data curation, final manuscript approval, formal analysis, funding acquisition, investigation, methodology, project administration, realization of operations and/or trials, resources, software, supervision, validation, visualization, writing - original draft preparation, writing - review & editing. |
REFERENCES
1. Vandeweyer E. Does liposuction influence lipidogram in females: in vivo study Aesthetic Plast Surg. 2002;26(1):17-9. PMID: 11891591
2. Sarici M, Demirseren ME, Durgun M, Ceran C, Yenidunya MO. Effects of reduction mammoplasty on metabolic profile and body weight. Aesthetic Plast Surg. 2011;35(6):995-9. DOI: http://dx.doi.org/10.1007/s00266-011-9719-7
3. Benatti FB, Lira FS, Oyama LM, do Nascimento CM, Lancha AH Jr. Strategies for reducing body fat mass: effects of liposuction and exercise on cardiovascular risk factors and adiposity. Diabetes Metab Syndr Obes. 2011;4:141-54. DOI: http://dx.doi.org/10.2147/DMSO.S12143
4. Robles-Cervantes JA, Yánez-Díaz S, Cárdenas-Camarena L. Modification of insulin, glucose and cholesterol levels in nonobese women undergoing liposuction: is liposuction metabolically safe? Ann Plast Surg. 2004;52(1):64-7. PMID: 14676702
5. Matarasso A, Kim RW, Kral JG. The impact of liposuction on body fat. Plast Reconstr Surg. 1998;102(5):1686-9. PMID: 9774031
6. Samdal F, Birkeland KI, Ose L, Amland PF. Effect of large-volume liposuction on sex hormones and glucose- and lipid metabolism in females. Aesthetic Plast Surg. 1995;19(2):131-5.
7. Capla JM, Rubin JP. Discussion: Prospective clinical study reveals significant reduction in triglyceride level and white blood cell count after liposuction and abdominoplasty and no change in cholesterol levels. Plast Reconstr Surg. 2011;128(3):198e-200e. PMID: 21865993
8. Benatti F, Solis M, Artioli G, Montag E, Painelli V, Saito F, et al. Liposuction induces a compensatory increase of visceral fat which is effectively counteracted by physical activity: a randomized trial. J Clin Endocrinol Metab. 2012;97(7):2388-95. DOI: 10.1210/jc.2012-1012
9. Yazigi Solis M, Artioli GG, Montag E, de Salles Painelli V, Saito FL, Lima FR, et al. The liposuction-induced effects on adiponectin and selected cytokines are not affected by exercise training in women. Int J Endocrinol. 2014;2014:315382. DOI: 10.1155/2014/315382
1. Hospital Daher Lago Sul, Brasília, DF,
Brazil
2. Clínica Di Lamartine e Galdino de Cirurgia
Plástica, Brasília, DF, Brazil.
Corresponding author: Jefferson Di Lamartine Galdino Amaral SCN QD.02, Torre A, Salas 1121 e 1123 - 11º Andar - “Shopping Liberty Mall” - Brasília, DF, Brazil Zip Code 70712-903 E-mail: jefferson@dilamartine.com.br
Article received: March 18, 2018.
Article accepted: November 11, 2018.
Conflicts of interest: none.









 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter