

Case Report - Year 2018 - Volume 33 -
Pyoderma gangrenosum following reduction mammoplasty: a case report
Pioderma gangrenoso em pós-operatório de mamoplastia redutora: relato de caso
ABSTRACT
Pyoderma gangrenosum (PG) is an inflammatory neutrophilic dermatosis of unknown etiology and is idiopathic in 25%- 50% cases. In approximately 50% of PG cases, an association with systemic diseases, such as Crohn's disease, monoclonal gammopathies, seropositive arthritis, collagenosis, Behcet's disease, Wegener's granulomatosis, and myeloproliferative and infectious diseases (mainly hepatitis and AIDS), has been described. Clinically, PG presents four variants: ulcerated, bullous, vegetative, and pustular. The most frequent form is ulcerative, which begins as a papule or nodule and evolves rapidly into ulcerated and painful lesions. In approximately 25% of PG cases, the onset of new lesions can be triggered by traumas such as insect bites, intravenous injections, and biopsy, a phenomenon known as pathergy. Here, we present a case of extensive PG of the breasts following reductive mammoplasty surgery. It was a difficult case to diagnose and was initiated in the postoperative period of another service. Due to the breakdown of the doctor-patient relationship, the patient approached us for assistance. The patient showed an excellent response to corticotherapy (intra and perilesional corticotherapy with triamcinolone) during debridement and oral steroid (prednisone) therapy in the weaning phase.
Keywords: Pyoderma gangrenosum; Mammoplasty; Reconstructive surgical procedures; Postoperative complications; Physician-patient relationship
RESUMO
O pioderma gangrenoso (PG) é uma dermatose neutrofílica inflamatória, de etiologia desconhecida. O PG é idiopático em 25-50% dos casos. Em aproximadamente 50% dos casos tem sido descrita a associação com doenças sistêmicas, tais como: doença de Crohn, gamopatias monoclonais, artrites soropositivas, colagenoses, doença de Behcet, granulomatose de Wegener, doenças mieloproliferativas e infecciosas, principalmente hepatites e Aids. Clinicamente, apresenta quatro variantes: ulcerada, bolhosa, vegetante e pustulosa. A forma mais frequente é a ulcerativa, que se inicia com pápula ou nódulo e evolui rapidamente para lesões ulceradas e dolorosas. Em até 25% dos casos de PG, o surgimento de novas lesões pode ser desencadeado por traumas, tais como picadas de insetos, injeções intravenosas e biópsia - fenômeno conhecido por patergia. Nesse trabalho, é apresentado um caso de PG extenso das mamas em pós-operatório de mamoplastia redutora, de difícil diagnóstico; iniciado na evolução pós-operatória em outro serviço. Devido à quebra da relação médico-paciente, vem procurar nosso serviço. Apresentou ótima resposta ao tratamento com corticoterapia (corticoterapia intra e perilesionais com triancinolona) no ato do desbridamento, e introdução de corticoterapia via oral (prednisona) em esquema escalonado de desmame.
Palavras-chave: Pioderma gangrenoso; Mamoplastia; Procedimentos cirúrgicos reconstrutivos; Complicações pós-operatórias; Relações médico-paciente
INTRODUCTION
Pyoderma gangrenosum (PG) is a neutrophilic inflammatory dermatosis of unknown etiology, which mainly affects adults aged between 20 and 50 years, and was described in 1930 by Brusting et al.1 PG is idiopathic in 25%-50% of cases. In approximately 50% of cases, its association with systemic diseases, such as Crohn’s disease, monoclonal gammopathies, seropositive arthritis, collagenosis, Behcet’s disease, Wegener’s granulomatosis, myeloproliferative and infectious diseases, mainly hepatitis and AIDS1-4, has been described.
Some patients with PG present altered cellular and humoral immunity with increased expression of interleukins, especially IL-8 and tumor necrosis factor. Clinically, it presents four variants: ulcerated, bullous, vegetative, and pustular. The most prevalent form is the ulcerative type, which begins as a papule or nodule and rapidly evolves into ulcerated and painful lesions4,5.
In up to 25% of PG cases, the appearance of new lesions can be triggered by traumas, such as insect bites, intravenous injections, and biopsy, a phenomenon known as pathergy6.
In this report, we present a case of extensive PG in the breasts in the postoperative period of reductive mammoplasty, which was difficult to diagnose, but showed excellent response to corticotherapy (intra and perilesional corticotherapy with triamcinolone) during debridement and oral steroid therapy (prednisone) in the weaning phase.
METHODS
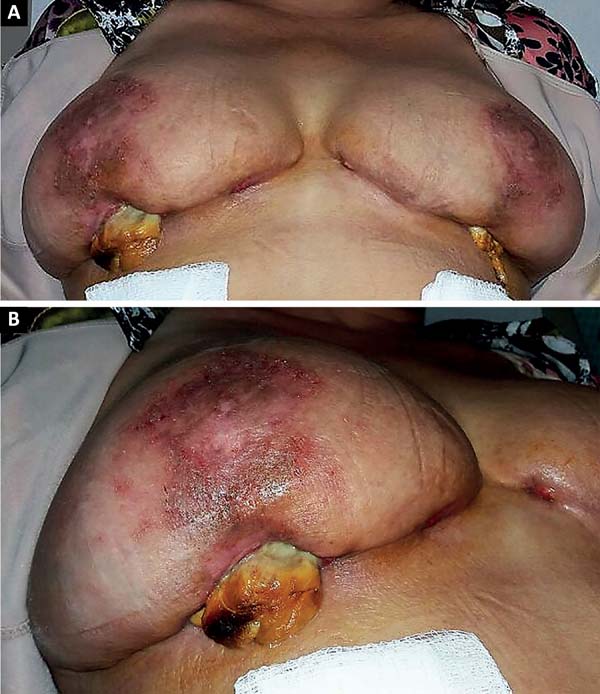
This is a case report of a 56-year-old female, who underwent reductive mammoplasty in another facility for esthetic reasons. Postoperatively, vesicles appeared around the surgical scars that subsequently ruptured and led to the emergence of hyperemic areas with ulceration. The wounds further progressed to areas of necrosis with exposure of breast tissue and purulent secretion. The surgical wound was bilaterally involved, and progressed beyond the borders of the surgical wound.
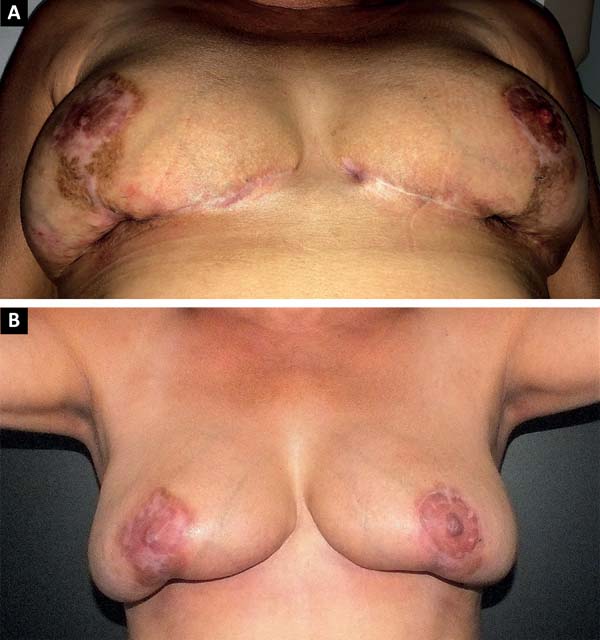
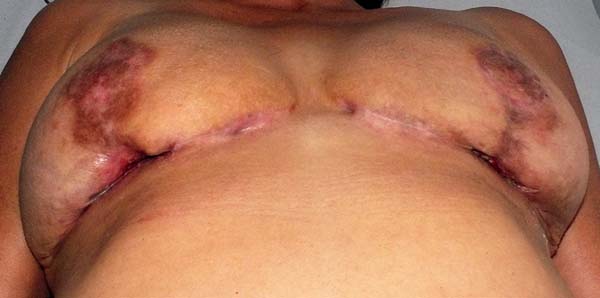
The patient was treated with oral antibiotic therapy by the attending physician present at that time. After three months of treatment with different antibiotic regimens, but no satisfactory results, and worsening of the overall condition, the patient sought our service (Figure 1A and 1B).
After a multidisciplinary discussion involving a dermatologist and an infectologist, debridement was initially proposed in a hospital environment with collection of multiple fragments of the breast tissue for diagnosis. Twelve fragments of tissues were collected in total (six fragments from each breast). Of the six fragments of each breast, we sent four fragments for culture and antibiogram and two for anatomopathological analysis.
At the end of the debridement and collection of the materials for the study, an empirical injection of 5 mL triamcinolone (20 mg/mL; diluted in 20 mL of SF 0.9%, totaling a solution of 25 mL) peri and intralesional, was divided for multiple applications to both breasts. As advised by the infectiologist, the antibiotic treatment was started orally in an empirical scheme immediately after debridement, until culture and pathology analyses were available to guide further (Figure 2).
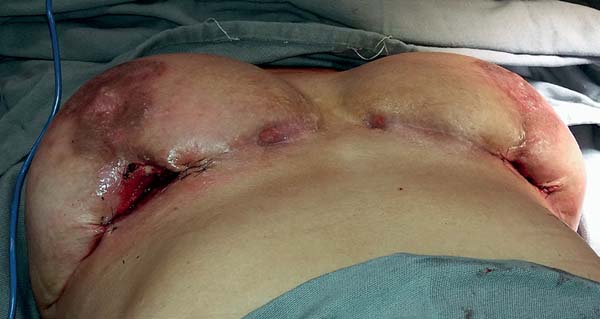
Suture closure was not performed; however, containment sutures were placed at the margins of the cavity (Figure 3). The debridement was also limited to the devitalized tissues, to avoid any further tissue damage.
RESULTS
Once the results of the cultures and anatomopathological analyses were obtained, the use of antibiotic was interrupted and oral corticotherapy was initiated, as the diagnosis of PG corroborated with the pathology result. The empirical scheme used for corticotherapy was as follows: starting with 20 mg prednisone for 1 week, followed by 15 mg for 1 week, 10 mg for another week, and 5 mg for 3 weeks, thus totaling 6 weeks of corticosteroid treatment in a phased approach (Figure 4A and 4B).
The lesions improved gradually after the first intraoperative injection of triamcinolone (Figure 5), suggesting that the condition was probably related to the patient’s immune system. Furthermore, since it was an infectious bacterial disease, the condition was expected to worsen when immunosuppressed with injectable and oral corticosteroids. The patient was investigated for autoimmune diseases, collagenosis, hepatitis, and HIV. Nevertheless, the research did not show any subclinical disease.
In the bloody areas, daily local care was followed with healing oils (Pielsana®) and gauze. Hyperbaric therapy was proposed for the patient, but she was unable to adhere to the treatment because of the expense. Therefore, no therapy session was performed in the hyperbaric chamber, but we believe that this would be an extremely valuable modality in the recovery of the wounded areas.
The patient was asked to continue the use of the post-surgical modeling bra that served as a support for the dressings, and the use of micropore or other adhesive tapes in contact with the skin was avoided to prevent any chances of subsequent trauma, even if minimal, in the vicinity of the wounds.
DISCUSSION
We are faced with a condition, relatively unknown, that can devastate a person’s life. A patient approaching a plastic surgeon to achieve an esthetic look for her breasts, and facing progressive and uncontrolled tissue destruction postoperatively, would be the exact opposite of what the patient expected.
The doctor-patient relationship is damaged due to lack of understanding between both the parties. The patient mistakenly believes it to be the surgeon’s technique error, while the surgeon believes it to be a surgical wound infection without proper diagnosis. This may lead to legal proceedings that can be a hassle for both individuals involved.
In the literature, the data of the PG disease is prevalent in the practice of plastic surgeons, dermatologists, and mainly wound care specialists. It is a rather uncommon disease, as revealed by a retrospective study conducted in Brazil showing an index of 0.38 cases per 10,000 visits. PG can be associated with other diseases, although 50% of cases arise alone6,7.
The diagnosis of PG is mainly clinical, since there are no specific serological or histological findings. The possibility of PG should be considered when destructive and painful necrotic ulcers complicate postoperative progress, especially in patients suffering from or had suffered from autoimmune disease, hepatitis, AIDS, myeloproliferative diseases, etc.
The rapid improvement of PG in several cases with the use of cyclosporine suggests the involvement of T-lymphocytes in its pathogenesis, since the main mechanism of action of this drug is the inhibition of T-lymphocyte activation, and reduction of interleukin-2 production. Cyclosporine also inhibits phagocytosis of neutrophils and monocytes, as well as the production of superoxide by polymorphonuclear cells. A similar mechanism (on T-lymphocytes) occurs with tacrolimus drug, which is also effective in the treatment of PG8.
The presented case showed quick improvement in six weeks of the injectable triamcinolone administration around and within the lesions, along with oral systemic corticotherapy (prednisone).
Although histopathology does not provide any specific findings and there is always the risk of aggravating the lesions by the pathergy mechanism, a biopsy can be useful. The most common findings are suppurative neutrophilic, necrotizing, and leukocytoclastic infiltration. Such characteristics, though non-specific, are additional evidence to the PG hypothesis. Culture and sensitivity tests may be useful in ruling out differential diagnosis involving fungi and atypical bacteria. Microbiological studies for diagnosing infections that complicate PG will also be useful 4-10.
Taking into consideration everything that was known about this case, two questions arise:
On the issues raised, we made the following observations: there are a large number of cases not yet diagnosed, and an even great number of non-reported cases. There is no record of plastic surgeons notifying about competent sanitary techniques. This could indicate that the information we have about PG today could probably be incorrect. It is necessary to review the case series on PG because this is a disease capable of destroying the patient’s life and harming the surgeon’s career.
The second question raised draws our attention to patients with underlying diseases who are not being treated. It is known from the literature that only 50% of the cases have a correlation with autoimmune diseases, hepatitis, AIDS, etc.; however, we can challenge this data.
The argument comes from the fact that plastic surgeons and dermatologists do not have the appropriate qualification to investigate a patient diagnosed with PG. It is often noted that after the problem is resolved, with or without correct diagnosis, and based on long-term dressings, the patient is discharged. On the other hand, due to the breakdown of the doctor-patient relationship, the patient does not return or follow the surgeon’s recommendations.
We understand that the professionals of internal medicine (general practitioner) and rheumatology are most suitable for the patient’s care after a diagnosis of PG. These professionals will be able to conduct correct subclinical investigation and exhaust the diagnosis possibilities.
The main message is that every postoperative patient with painful ulcers that progress even during the course of antibiotics, should be checked for PG diagnosis. A biopsy is recommended, and the pathologist may not include the diagnosis of PG in the report; however, if the description of the histological findings corresponds to neutrophilic infiltration, the surgeon should definitely consider PG as the diagnosis.
A patient of this kind should be investigated by a general practitioner or a qualified specialist (in our opinion, a rheumatologist would be the best specialty for this research) to explore the comorbidities that may be present subclinically. If the patient’s condition improves with systemic corticotherapy, it indicates a case of PG.
REFERENCES
1. Meyer TN. Pioderma Gangrenoso: Grave e Mal Conhecida Complicação da Cicatrização. Rev Bras Cir Plást. 2006;21(2):120-4.
2. Soares JM, Rinaldi AE, Taffo E, Alves DG, Cutait RCC, Estrada EOD. Pioderma gangrenoso pós-mamoplastia redutora: relato de caso e discussão. Rev Bras Cir Plást. 2013;28(3):511-4.
3. Blitz NM, Rudikoff D. Pyoderma gangrenosum. Mt Sinai J Med. 2001;68(4-5):287-97.
4. Wines N, Wines M, Ryman W. Understanding pyoderma gangrenosum: a review. Med Gen Med. 2001;3(3):6.
5. Wollina U. Clinical management of pyoderma gangrenosum. Am J Clin Dermatol. 2002;3(3):149-58.
6. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34(3):395-409.
7. Souza CS, Chioss MPV, Takada MH, Foss NT, Roselino AMF. Pioderma gangrenoso: casuística e revisão de aspectos clínico-laboratoriais e terapêuticos. An Bras Dermatol. 1999;74(5):465-72
8. Schöfer H, Baur S. Successful treatment of postoperative pyoderma gangrenosum with cyclosporin. J Eur Acad Dermatol Venereol. 2002;16(2):148-51.
9. Born S, Marsch WC. Postoperative pyoderma gangrenosum. Chirurg. 2001;72(9):1043-7.
10. Baruch J, Julien M, Touraine R, Slaoui SE, Auffret P. Postoperative pyoderma gangrenosum and cancer of the breast. Apropos of a case. Chirurgie. 1989;115(2):142-5.
1. Faculdade de Medicina de Jundiaí, Jundiaí, SP,
Brazil.
2. Clínica Particular, Dermatologia e Medicina
Interna, Osasco, SP, Brazil.
Corresponding author: Carlos Jose Gaspar-Junior, Rua Ana Pereira Melo, nº 253 - Sala 1010 - Vila Campesina - Osasco, SP, Brazil, Zip Code 06028-030. E-mail: dr.gaspar.jr@gmail.com / clinica.gaspar@outlook.com
Article received: May 15, 2018.
Article accepted: October 1, 2018.
Conflicts of interest: none.




 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter