

Original Article - Year 2018 - Volume 33 -
Facial rejuvenation with fat grafting: systematization and study of 151 consecutive cases
Rejuvenescimento facial com lipoenxertia: sistematização e estudo de 151 casos consecutivos
ABSTRACT
Introduction: Considering that the loss of facial volume is a primary factor associated with aging, an increased demand for safe, long-lasting, and biocompatible filling materials has been observed. Thus, the use of fat grafting has gained considerable popularity. However, there are open questions about the safety, efficacy, and durability of fat grafting. Moreover, most studies have not presented the volumes injected in each region, making learning challenging for beginners in the area. In this study, the results of facial rejuvenation with fat grafting in 151 consecutive cases were analyzed.
Methods: Fat was collected via manual suction, centrifuged at 448 g (2000 rpm/radius 10 cm) for 4 min, and injected with microcannulas that are 1-1.1 mm in size. The injection sites and corresponding injection volumes were identified.
Results: The mean follow-up time was 289.29 days (minimum: 7 days, maximum: 1254 days, and standard deviation [SD]: 275.1), and the mean injection volume was 32 mL (range: 4-68 mL, SD: 14). Moreover, no complications were observed.
Conclusion: Fat grafting is a safe, predictable, and effective procedure, and it can be used for facial rejuvenation in certain cases.
Keywords: Face; Rhytidoplasty; Autologous transplantation; Subcutaneous fat; Aesthetics; Reconstructive surgical procedures
RESUMO
Introdução: Considerando que a perda de volume facial é fator primário de envelhecimento,
tem acontecido um aumento da demanda por materiais de preenchimento que
sejam seguros, de longa duração e biocompatíveis. Nesse sentido, a
utilização do enxerto de gordura vem ganhando bastante popularidade.
Entretanto, existem questionamentos sobre segurança, eficácia e durabilidade
da lipoenxertia. Além disso, a maioria dos artigos da literatura não
menciona volumes injetados em cada área, dificultando o aprendizado dos
iniciantes. Nesse estudo, analisam-se os resultados de uma série de 151
casos consecutivos de rejuvenescimento facial com lipoenxertia.
Métodos: A gordura foi colhida por meio de sucção manual, centrifugada a 448g (2000
rpm/ raio 10cm) por 4 minutos e injetada com microcânulas de 1 a 1,1mm.
Descreve-se a sistematização de áreas de injeção, com os respectivos volumes
a serem aplicados.
Resultados: Encontrou-se seguimento médio de 289,29 dias (mínimo 7, máximo 1254, DP
275,1), o volume médio injetado foi de 32 ml, variando de 4 a 68 (DP 14).
Não houve complicações.
Conclusão: A lipoenxertia é um procedimento seguro, previsível e efetivo, como opção de
tratamento, para rejuvenescimento facial, em determinados casos.
Palavras-chave: Face; Ritidoplastia; Transplante autólogo; Gordura subcutânea; Estética; Procedimentos cirúrgicos reconstrutivos
INTRODUCTION
Evidence of volume loss as a primary factor associated with aging was clearly described by Lambros1. Thus, the demand for safe, long-lasting, and biocompatible filling materials has been increasing2. Autologous fat grafts have been widely used by plastic surgeons because they are abundant and easily available3.
However, the lack of reliability and consistency in terms of its final clinical outcome is a major concern, which may result in the need for multiple procedures3. In fact, the literature has not provided reliable data on how much from the injected fat remains in the body4. This variability and the poor results published in the late 1980s and early 1990s reinforced that several surgeons believe that the use of fat as a filling material is not reliable5,6.
Since the late 1980s, Coleman7 has advocated for the thorough systematization of fat grafting as a solution for minimizing its lack of reliability. Even authors who had already published unsatisfactory results about fat grafting began to use the methods published by Coleman and had obtained satisfactory results, indicating that the procedure may be successful if the transplanted fat is cautiously managed8,9.
Coleman has continually obtained expressive, consistent, and long-term results, and in the mid-1990s, he began to consider other fat attributes, which resulted in improved skin quality, smoothing of wrinkles, decreased pores, and improved pigmentation9. Tonnard et al. have reinforced this clinical impression and published studies about intradermal fat injection using a thin needle (Sharp-Needle Intradermal Fat - SNIF) and fat nanografting using even thinner needles10,11.
The present study aimed to analyze the results of the technique described by Coleman and to reinforce the systematization of the method for the modification of facial contour. Moreover, we used the methodology described in detail by Lam et al. in the book Complementary Fat Grafting12.
OBJECTIVE
This study aimed to analyze the results of the abovementioned technique and to present the injection volumes used in a series of cases.
METHODS
This is a retrospective clinical trial based on the medical records of all patients who underwent surgery in this institution between October 14, 2014 (which is the time we started using the methodology of facial fat grafting) and September 14, 2017 in Fortaleza, Ceará, Brazil. All patients undergoing face fat grafting were included. Only one surgeon conducted the surgery in all patients. However, patients undergoing fat grafting for reasons other than changing the contour of the face, such as exclusive treatment of acne scars, were not included. The study was conducted in accordance with the declaration of Helsinki, and informed consent was obtained from all patients.
Description of the technique: fat collection
Donor area
The removal area was chosen according to convenience due to the ease of positioning, abundance of material, and body contour of the patient. Thus, the most frequent areas were the flank and trochanteric region. Patients undergoing abdominoplasty had the region to be excised as a donor area of choice.
Removal method
Patients undergoing regional blocks for simultaneous body procedures had the donor area infiltrated with 0.9% NaCl solution and 1:200,000 adrenaline solution. Approximately 0.4% lidocaine and 0.01% levobupivacaine were added in patients who did not receive anesthetic block. The infiltrated volume approximately corresponds to the volume for removal.
Fat was removed via suctioning with a 3-mm cannula with 16 1-mm holes and sharp edges (Fagha Medical) coupled to a 10-mL Luer lock syringe (BD Medical). The plunger was pushed by hand aiming to maintain 1 mL of negative pressure.
The only difference between this technique and the one described by Lam et al.12 is the non-use of albumin solution and utilization of cannulas from another manufacturer, with minor differences.
Preparation
The filled syringes were subjected to decanting, whereas the others were removed. According to the guideline, the syringes that were first subjected rapidly decanted a clear infranate, which was discarded, and the syringe was filled again by the surgeon.
The syringes were closed and centrifuged (Surgical Monserrat®, R = 100 mm) at 2000 rpm (448 g) for 4 min. Then, the infranate and supranate were discarded. A gauze was placed in contact with the top of the fat to absorb residual oil.
The remaining fat in the syringe was transferred to another syringe to achieve a volume of 10 mL. The fat was slightly homogenized and mixed between two syringes using a transfer device. Then, prior to the application of fat, it was again transferred to 1-mL syringes.
Preparation of the receiving area
Patients who underwent body procedures, rhytidoplasty, and other simultaneous face procedures were sedated. Those who underwent rhinoplasty received general anesthesia. Furthermore, only local anesthesia was used in some cases of isolated fat grafting.
In all cases, blockade of the infraorbital and facial zygomatic branches of the trigeminal nerve was performed. Receiving areas were infiltrated with 0.9% NaCl solution, adrenaline 1:200.00 and lidocaine 0.4% with the same cannulas that will be used to inject fat. In cases of rhytidoplasty, the solution had an adrenaline concentration of 1:400.000.
Fat infiltration
The cannulas have 1- or 1.1-mm light and length from 3 to 7 cm (Rhosse Instruments®). We only used straight cannulas coupled to 1-mL syringes (BD Medical®).
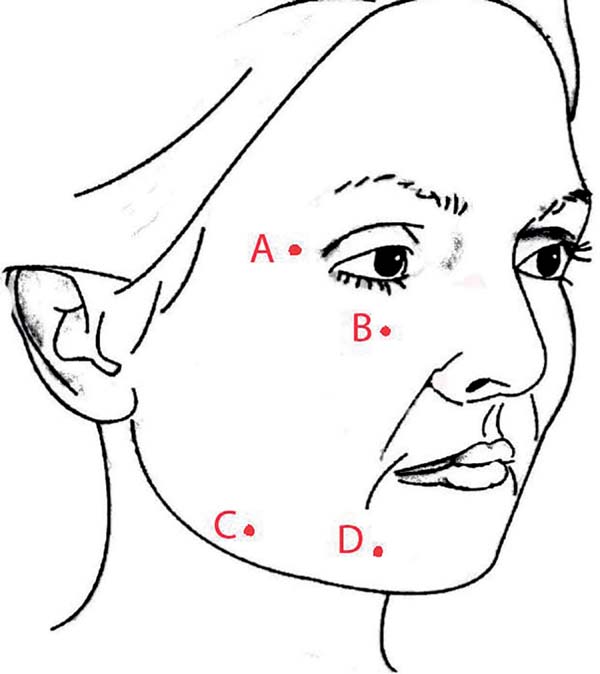
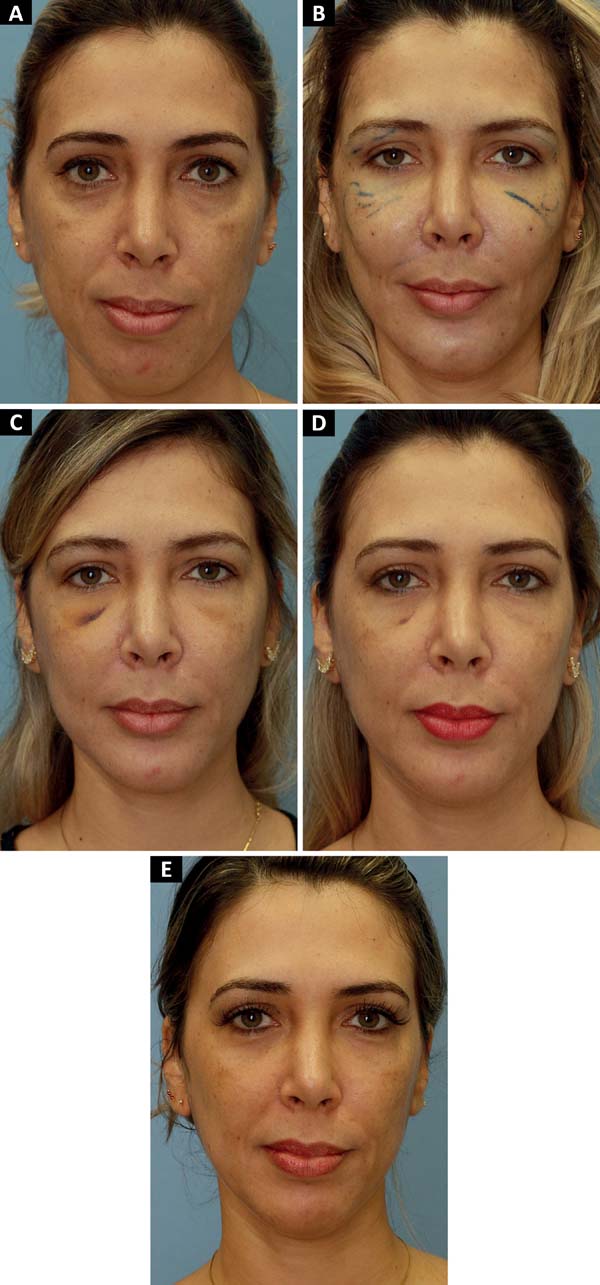
The entry points of the cannula were punctured with a 40×12 cutting needle at routine locations A, B, C, and D, as shown in Figure 1. Further punctures were made as needed in each case. However, there was no need to close the incisions.
Areas of application
The preparation and application methodology was in accordance with that described by Lam et al.12. Both the anterograde and retrograde techniques were used with the injection of approximately 0.1 mL every 1 cm of cannula displacement. The areas of application are presented in Figure 2.
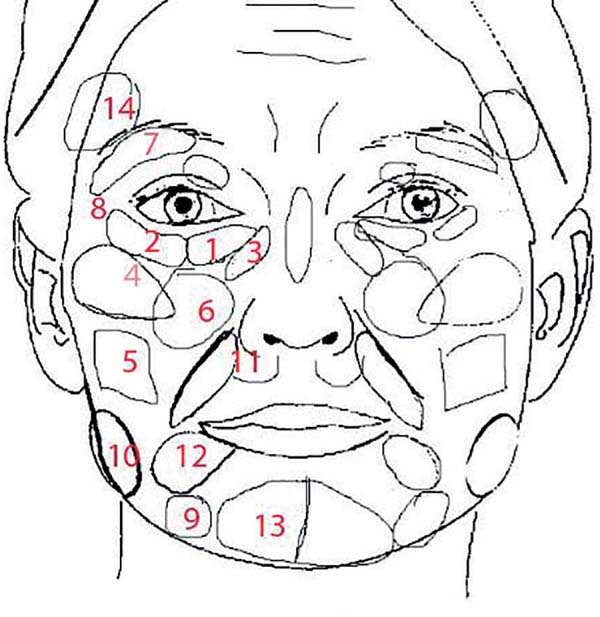
A spreadsheet with the regions, application plan, and entry points as well as the expected volume expected and that used in each area was created (Table 1). A room assistant strictly followed the application and took note of the areas and volumes.
| Region | Injected volume (mL) | ||||||
|---|---|---|---|---|---|---|---|
| Acess | Right | Left | Treatment frequency of region | ||||
| Planned | Accomplished (average / SD) | Planned | Accomplished (average / SD) | ||||
| Medial LOM* (supraperiosteal) | A | 1 | 1.0/0.1 | 1 | 1.0/0.1 | 130 | 92.2% |
| Lateral LOM* (supraperiosteal) | A | 1 | 1.0/0.1 | 1 | 1.0/0.1 | 129 | 91.5% |
| Nasojugal fossa (supraperiosteal) | A | 1 | 1.0/0.2 | 1 | 1.0/0.2 | 126 | 89.4% |
| Lateral zygomatic (three planes) | A | 2 | 2.1/0.5 | 2 | 2.1/0.5 | 122 | 86.5% |
| Buccal region (subcutaneous) | A | 2 | 2.3/0.8 | 2 | 2.2/0.8 | 42 | 29.8% |
| Anterior zygomatic (three planes) | B | 3 | 3.1/0.9 | 3 | 3.1/0.9 | 125 | 88.7% |
| UOM ** (upper orbital margin) | B | 1 | 1.0/0.6 | 1 | 1.0/0.6 | 26 | 32.6% |
| Lateral palpebral angle (supraperiosteal) | B | 0.5 | 0.7/0.7 | 0.5 | 0.7/0.7 | 29 | 27.7% |
| Mandibular groove (three planes) | C | 3 | 2.3/0.5 | 3 | 2.3/0.5 | 66 | 46.8% |
| Lateral mandible (deep subcutaneous) | C | 6 | 4.9/1.3 | 6 | 4.8/1.2 | 82 | 58.2% |
| Pre-canine Fossa (deep and intermediate) | A | 1.5 | 1.7/0.3 | 1.5 | 1.7/0.3 | 112 | 79.4% |
| Marionette (superficial subdermal) | D | 1.5 | 1.5/0.6 | 1.5 | 1.5/0.6 | 31 | 22.0% |
| Chin | D | 3 | 2.9/0.6 | 3 | 2.9/0.6 | 69 | 48.9% |
| Temporal region | B | 2 | 2.9/1.7 | 2 | 2.9/1.7 | 16 | 11.3% |
RESULTS
A total of 151 face fat grafting procedures were performed between October 14, 2014 and September 14, 2017, of which 148 aimed to modify the face contour. We did not include two cases involving treatment of acne scars and one case of correction of Bichat ball atrophy. Approximately, 8.8% (n=13) and 91.2% (n=135) of the patients were men and women, respectively. The age of the patients ranged from 22 to 82 years (mean: 45.45 years, standard deviation [SD]: 12.33). A total of 87 (59.2%) patients underwent body surgery, and 63 (42.9%) had face surgery. The number of patients are shown in Table 2.
| Rhinoplasty | 23 | 36.5% |
| Rhytidoplasty | 18 | 28.6% |
| Forehead lift | 14 | 22.2% |
| Upper blepharoplasty | 11 | 17.5% |
| Mentoplasty | 5 | 7.9% |
| Buccal fat extraction | 4 | 6.3% |
| Lower blepharoplasty | 1 | 1.6% |
| Otoplasty | 1 | 1.6% |
All patients were followed-up at least until the seventh postoperative day. Four (2.7%) patients were lost to follow-up after 7 days due to unknown reasons. The mean follow-up time was 289.29 days (minimum: 7 days, maximum: 1254 days, and SD: 275.1).
Three (2%) patients underwent a second fat grafting procedure because they wanted a more visible result. The mean interval between the first and second procedure was 16 mos (SD: 5.8). One of these three patients had already undergone face fat grafting before the start of this study.
No complications correlated to the method were observed, considering that the presence of ecchymosis and edema within the first 15 days is not a complication but normal side effects of the procedure. Previously non-existent asymmetries were not observed.
Accurate information about the injected volumes in 7 (4.7%) of 148 patients was not obtained. Thus, the injected volume statistics were calculated considering a total of 141 patients. Table 2 shows the distribution of volumes in their respective regions with the mean injected volume and frequency with which this area was treated. The mean injected volume was 32 mL (range: 4-68 mL, SD: 14).
DISCUSSION
Considering that volume loss is a primary factor associated with aging1, we have adopted systematic volumization in our clinical practice in recent years. This demands the assessment of safe, long-lasting, and biocompatible materials, as stated by several authors in the literature2.
Several types of synthetic filling materials, with emphasis on hyaluronic acid, are available13. However, none of the materials are perfect. Thus, although some publications have been discouraging this procedure in the past5,6, we have observed that such a procedure has been used again for autologous fat grafting for soft tissue reconstruction within the last 20 years, and for a wide variety of indications, such as facial rejuvenation3.
However, even with the increased use of fat grafting, high-quality clinical studies of any of the technical stages of the procedure, such as the selection of the ideal donor area, fat collection, processing, and injection technique, are extremely limited14. Moreover, previous studies have rarely shown the injected volumes in each area, which makes learning difficult for beginners in the area.
Thus, we chose to follow a feasible and reproducible methodology and create a basic planning model that is safe for beginners and most patients. We applied similar volumes in the same region in different patients, even though the need for filling varied among individuals. That is, due to the treatment of more or fewer areas, the differences in the total injected volume (minimum of 4 mL and maximum of 68 mL) were higher than those in the injected volume in the same region in different individuals.
This study aimed to identify safety measures and procedures applicable to most cases. To date, it is necessary to explain that the procedure may not be sufficient and that the procedure, in all or any of its stages, can be repeated.
Surgeons are usually doubtful of the reliability, durability, and safety of facial fat grafting. Thus, we analyzed a series of cases considering these points.
There are several definitions for the term reliability. Cronbach defined it in four ways: “Definition 1 - coefficient of stability: Reliability is the degree to which the result of a test remains with unalterable differences individually in any treatment”15. In an actual setting, we believe that reliability is achieving predictable results. That is, there are no cases in which individuals had more or less grip of the graft, particularly in the differences between the sides of the face, causing asymmetries.
In this regard, since previously non-existent asymmetries have not been observed, no significant variability was noted in each case. Tools that objectively determine the variability of the grip in different cases are not available. However, in all patients, the treated area improved, and there were no reports of excessive treatment. However, cases of insufficient treatment have been reported.
Therefore, the procedure, as previously described, satisfied the expected degree of reliability. However, further studies must be conducted to objectively validate its reliability.
Expectations in terms of short- and long-term evolution with regard to durability must be explained in detail.
Appearance significantly changed within the first week when a patient begins to feel uncomfortable appearing in public. After 1 week, most patients were highly satisfied with themselves. However, they asked whether they were still extremely swollen. After 30 days, the result was still slightly voluminous, but it was already close to the result after 90 days.
We considered the result obtained after 90 days as the final result because minimal changes were observed after this period, although the study that was the basis for our method has stated that edema should resolve around the sixth postoperative month. The same author has observed some increase in volume in several patients after 1 year12.
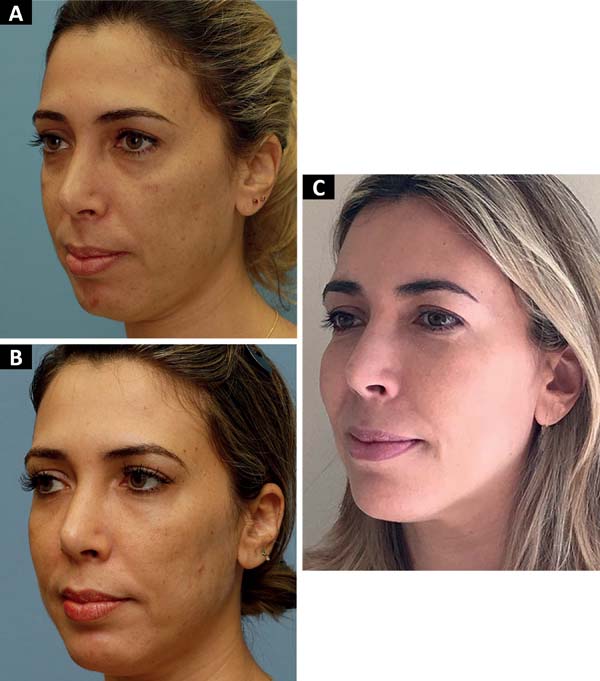
We agree with this statement as we also noticed an increase in the result without loss of volume until the current follow-up (maximum of 1254 days), as shown in Figures 3 and 4, in which the zygomatic increase persists. However, we could not objectively quantify this. Thus, such results should be validated in future studies.
However, when we identified the durability of the method, it does not answer the question on how much remains from what was injected. To date, a measurement methodology capable of answering this question is not available. Therefore, we prefer to direct our learning in understanding the volume required to correct each deformity.
For example, we observed that a volume of 2-3 mL is sufficient to correct the lower orbital margin region and that the lateral mandible does not undergo major transformations with a volume less than 4 mL.
With regard to graft survival, theories about the survival mechanism of injected fat, which is not consistent in the literature, are worth discussing.
In 1923, Neuhof and Hirshfield have observed that fat grafts at 2-3 months were dominated by degenerative phenomena. Moreover, some degree of regeneration during the second month was observed, and by the end of 5 months, regeneration was complete and a new metaplastic fat assumed the appearance of adipose tissues permeated by connective tissues. Then, they have concluded that the injected fat died completely and was replaced by fibrous tissues or neoformed metaplastic adipose tissues. Thus, this introduced the host cell replacement theory, or reposition theory9.
Thirty years later, Peer16 has concluded that approximately 50% of adipose tissues survived, giving rise to the theory of cell survival.
According to Coleman9, both theories , in addition to the role of undifferentiated cells, may be correct, and this has been considerably explored to date.
Several studies on the longevity of grafts have been conducted. However, research about their complications is limited. Lam et al.12 have reported about possible complications, such as protuberances, bulging, persistent zygomatic edema, overcorrection, under-correction, and retraction at the injection site. Among these complications, only under-correction was observed. Therefore, our procedure was considered safe.
A recent study has shown that age affects the viability of adipocytes in specific anatomical regions in different ways and that the lower abdomen is preferred than the flank in patients under 45 years of age. Meanwhile, the flank is preferred to the lower abdomen in patients over 45 years of age. In this study, no difference was observed in the viability of the inner surface of the thigh in the two age groups14. Therefore, in the future, we may consider the age of the patient when choosing the donor area. However, to date, practicality and availability are still considered more relevant attributes.
CONCLUSION
Fat grafting is a safe, predictable, and effective procedure, and it can be used in facial rejuvenation in certain cases.
COLLABORATIONS
|
EATF |
Data analysis and/or interpretation; statistical analysis; final approval of the manuscript; data collection; conceptualization; conception and design of the study; resource management; project management; research; methodology; performance of surgeries and/or experiments; writing and preparation of the original manuscript (writing; review; editing; supervision; and visualization). |
|
DBS |
Statistical analysis; data collection; and writing and preparation of the original manuscript. |
REFERENCES
1. Lambros V. Observations on periorbital and midface aging. Plast Reconstr Surg. 2007;120(5):1367-76.
2. Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 Suppl):7S-14S.
3. Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130(1):249-58.
4. Fontdevila J, Serra-Renom JM, Raigosa M, Berenguer J, Guisantes E, Prades E, et al. Assessing the long-term viability of facial fat grafts: an objective measure using computed tomography. Aesthet Surg J. 2008;28(4):380-6.
5. Chajchir A. Fat injection: long-term follow-up. Aesthetic Plast Surg. 1996;20(4):291-6.
6. Ersek RA. Transplantation of purified autologous fat: a 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87(2):219-27.
7. Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg. 1997;24(2):347-67.
8. Ersek RA, Chang P, Salisbury MA. Lipo layering of autologous fat: an improved technique with promising results. Plast Reconstr Surg. 1998;101(3):820-6.
9. Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(3 Suppl):108S-20S.
10. Zeltzer AA, Tonnard PL, Verpaele AM. Sharp-needle intradermal fat grafting (SNIF). Aesthet Surg J. 2012;32(5):554-61.
11. Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132(4):1017-26.
12. Lam SM, Glasgold MJ, Glasgold RA. Complementary Fat Grafting. Philadelphia: Lippincott Williams & Williams; 2007.
13. Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009;25(2):86-94.
14. Geissler PJ, Davis K, Roostaeian J, Unger J, Huang J, Rohrich RJ. Improving fat transfer viability: the role of aging, body mass index, and harvest site. Plast Reconstr Surg. 2014;134(2):227-32.
15. Cronbach LJ. Test reliability; its meaning and determination. Psychometrika. 1947;12(1):1-6.
16. Peer LA. Cell survival theory versus replacement theory. Plast Reconstr Surg (1946). 1955;16(3):161-8.
1. Clínica Eduardo Furlani, Cirurgia Plástica,
Fortaleza, CE, Brazil.
2. Universidade Federal do Ceará, Medicina,
Fortaleza, CE, Brazil.
Corresponding author: Eduardo Antonio Torres Furlani, Rua Barbosa de Freitas, nº 1990 - Aldeota - Fortaleza, CE, Brazil, Zip Code 60170-021. E-mail: eduardo@eduardofurlani.com.br
Article received: May 23, 2018.
Article accepted: October 4, 2018.
Conflicts of interest: none.





 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter