

Case Report - Year 2018 - Volume 33 -
Pyoderma gangrenosum: a challenge for the plastic surgeon
Pioderma gangrenoso: um desafio para o cirurgião plástico
ABSTRACT
Introduction: Pyoderma gangrenosum (PG) is a chronic and
rare autoimmune dermatosis. Its etiology remains poorly
understood, being idiopathic in 25 to 50% of cases; in others, it is
associated with systemic diseases with autoimmune background
and has an incidence of 2 to 3 cases per 1 million per year. In
Brazil, the rate is 0.38 cases per 10,000 clinical visits, and women
between the second and fifth decades of life are the most affected.
The clinical presentation is variable, and the ulcerous form,
which appears on a previous scar, is the most prevalent.
Case Report: A 39-year-old, previously healthy female underwent
reduction mammoplasty, and later developed a necrotic ulcer
on a vertical left breast scar. Debridement of devitalized
tissue was performed, with significant worsening despite
antibiotic therapy. The appearance suggested PG. Treatment
with oral and topical corticosteroids was then initiated
with remission.
Conclusions: PG represents a diagnostic
challenge, and can be confused with surgical site infection.
Keywords: Pyoderma gangrenosum; Mammoplasty; Corticosteroids; Reconstructive surgical procedures; Immunotherapy
RESUMO
Introdução: O pioderma gangrenoso (PG) corresponde a uma dermatose autoimune crônica e
rara. Sua base etiológica ainda permanece pouco conhecida, sendo idiopático
em 25 a 50% dos casos, nos demais está associado com doenças sistêmicas de
fundo autoimune, tem uma incidência de 2 a 3 casos em 1 milhão de habitantes
por ano. No Brasil, este índice é de 0,38 casos por 10.000 atendimentos, as
mais acometidas são as mulheres entre a segunda e quinta década de vida. O
quadro clínico é variável, sendo que a forma ulcerosa, que surge sobre uma
cicatriz prévia, é a mais prevalente.
Relato de Caso: Paciente do sexo feminino, 39 anos de idade, previamente hígida, foi
submetida à mamoplastia redutora, evoluiu com úlcera necrótica em cicatriz
vertical de mama esquerda. Realizado desbridamento de tecidos
desvitalizados, prescrita antibioticoterapia, apresentando piora importante
da lesão, sendo considerada a hipótese de PG. Iniciado tratamento com
corticoterapia oral e tópica com remissão do quadro.
Conclusões: O PG representa um desafio no diagnóstico e, geralmente, demonstra a
dificuldade diagnóstica, podendo ser confundido com infecção do sítio
cirúrgico.
Palavras-chave: Pioderma gangrenoso; Mamoplastia; Corticosteroides; Procedimentos cirúrgicos reconstrutivos; Imunoterapia
INTRODUCTION
Pyoderma gangrenosum (PG) is a chronic and rare autoimmune dermatosis, first described by Brunsting and O Leary in 1930, highlighting the absence of an infectious nature. Histopathologically, it is characterized by a nonspecific, noninfectious, non-neoplastic dermal neutrophilic infiltrate, without evidence of primary vasculitis1.
The etiological basis remains little understood, being idiopathic in 25 to 50% of cases. Other cases are associated with systemic autoimmune diseases, especially inflammatory bowel disease and mainly ulcerative colitis, but also arthritis, IgA gammopathy, and others2,3. It may also appear as a paraneoplastic manifestation or after the use of certain medications (propylthiouracil and isotretinoin in particular) and illicit substances such as cocaine4.
PG has an incidence of 2 to 3 cases per 1 million per year4,5. National data from a retrospective analysis indicated that in Brazil, this rate is 0.38 cases per 10,000 clinical visits5, with women between the second and fifth decades of life being most affected4.
The clinical presentation is variable, and a single or multiple ulcerous form, which appears on a prior scar, is the most prevalent6. The ulcers are well circumscribed, and have a violaceous halo and necrotic-hemorrhagic center, with characteristic purulent and accelerated centrifugal growth, ending with accelerated formation of granulation tissue7. In addition to the ulcerated form, PG has pustular and vegetative forms, which are less prevalent and have fewer postoperative complications8.
Histopathology and immunohistochemistry in PG are nonspecific, and no serological markers are available for laboratory diagnosis; thus, diagnosis is clinical by default9,10.
Knowledge of the pattern of the cutaneous lesion is important, because diagnosis of post-surgical PG can be delayed. More commonly suspected diagnoses such as wound dehiscence and infection result in unnecessary debridement that tends to worsen the clinical presentation, since the pattern of the PG lesion is related to the phenomenon of pathergy in up to 50% of cases, with minor trauma triggering new lesions6,11.
The plastic surgeon should include the diagnosis of PG in the differential diagnosis, since the knowledge of cutaneous lesions, predisposing factors, and surgical risk factors enables avoidance of exacerbation.
CASE REPORT
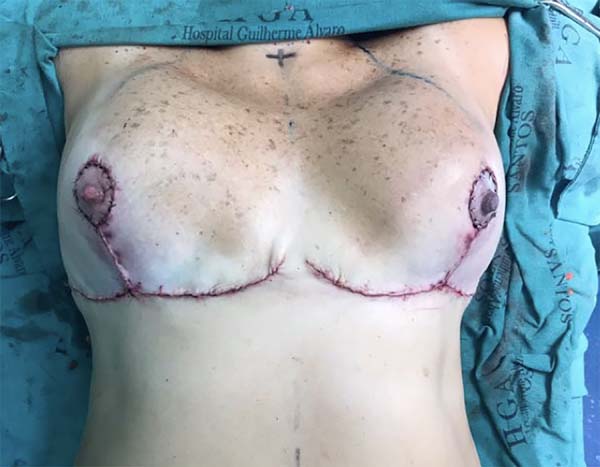
A 39-year-old, previously healthy Caucasian patient, with no surgical or obstetrical history, underwent reduction mammoplasty (Figure 1), and was discharged after 24 hours without complaints and in good general condition.
The patient received antibiotic prophylaxis with cefazolin 1 g every 6 h during hospitalization, followed by cephalexin 500 mg every 6 h, until the postoperative day (POD) 7. She was reassessed 72 h after the surgical procedure, still without complaints. The surgical wound had a good appearance, and a micropore dressing was in place.
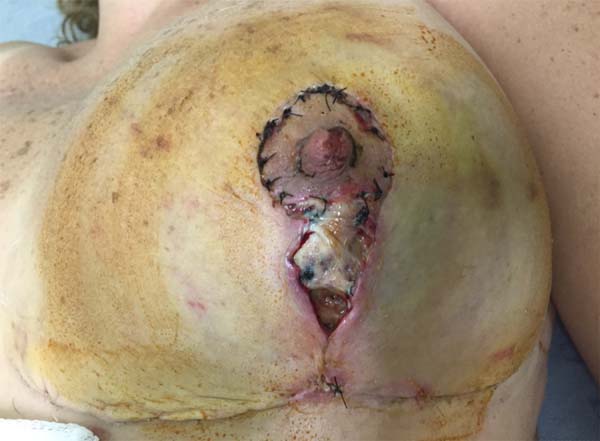
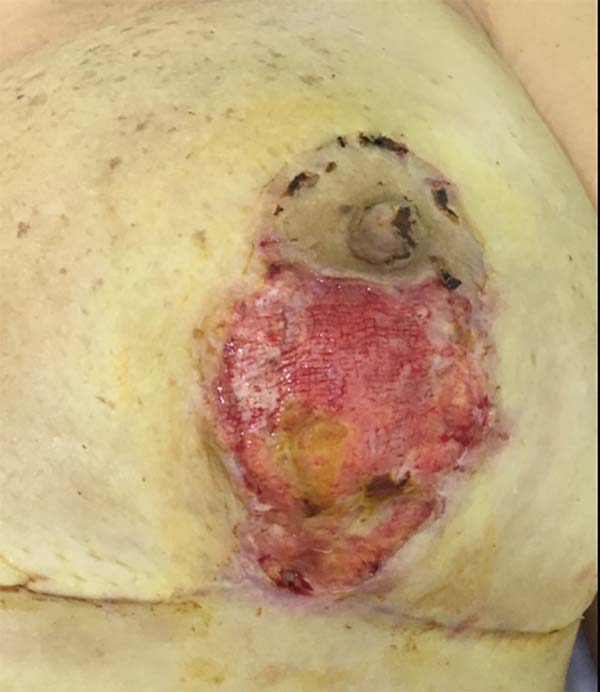
On 15 POD 15, during routine follow-up, the surgical wound presented a necrotic ulcer in a vertical scar on the left breast, with drainage of moderate seropurulent secretions (Figure 2). Debridement of devitalized tissue was performed, together with antibiotic therapy with ciprofloxacin 500 mg every 12 h and local dressings with collagenase ointment, in addition to micropore dressings. The wound initially appeared improved. On POD 17, the patient was reevaluated for significant worsening, with centripetal progression, affecting segments of the scar in the inframammary groove. The patient denied systemic symptoms.
Due to the surprising change, PG was considered. Consequently, a systematic review was performed in the literature regarding the therapeutic management of PG (Table 1).
| Database | Search strategy | Results | Clinical Cases |
|---|---|---|---|
| PubMed | (pyoderma gangrenosum) AND (breast) | 81 | 66 |
| Lilacs | (pyoderma gangrenosum) AND (breast) | 3 | 3 |
| SciELO | (pyoderma gangrenosum) AND (breast) | 2 | 2 |
| Total | 86 | 71 |
First-line treatment was initiated with oral and topical corticosteroids. Prednisone was administered at 60 mg/day for 7 days, 40 mg/day for 7 days, 20 mg/day for 7 days, and finally 10 mg/day for 4 days, ending with 10 mg/day on alternate days, for a total of 28 days (Figure 3).
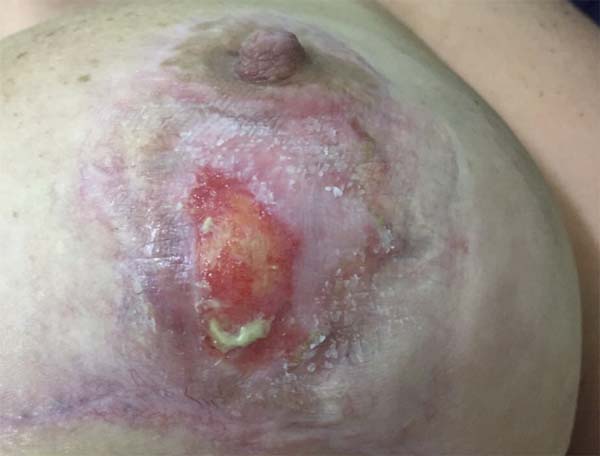
The topical treatment comprised daily fludroxycortide 0.125 mg/g (Drenison®), maintained from the beginning of oral therapy until the consolidation of the scar, which occurred at the end of the 2nd postoperative month, approximately 20 days after the end of oral corticosteroid therapy (Figure 4). The patient had a favorable course, with complete closure of the lesions after treatment for 1 month. Figure 5 shows the appearance in the 3rd postoperative month.
DISCUSSION
PG is a devastating complication both for the patient and the plastic surgeon, leading to questions about the technical quality of the surgical procedure12. The absence of a supplemental exam that confirms the diagnosis of PG, combined with nonspecific findings on histopathology, requires clinical knowledge of this disease to enable diagnosis1,2,4.
The objective of treatment is to limit tissue destruction, promote healing, and obtain a good esthetic result. Debridement and skin grafts are contraindicated3-5.
First-line treatment with systemic corticosteroids is the most effective option for PG. Immunosuppressive doses are necessary in the majority of cases, with approximately 100-200 mg/day of prednisolone or 60-80 mg/day of prednisone 4,6,7.
Alternatively, cyclosporine, at doses of 6-10 mg/kg/day, can produce significant improvement, with healing in 1 to 3 months, and is indicated for a minority of patients who do not respond to steroid therapy7,8.
TNF-alpha inhibitors, such as infliximab, show good results in some patients10,12.
Hyperbaric oxygen therapy may be indicated for patients who cannot tolerate or do not respond to high doses of systemic corticosteroids; however, the therapeutic result is less effective, as demonstrated in some case series11. Topical therapy is indicated to complement systemic therapy; corticosteroids alone are the drugs of choice in selected cases of lesser severity6,7,11.
Antibiotic therapy is not supported for PG cases, as demonstrated in all series studied; therefore, there are no clinical benefits from the use of antimicrobial agents in these patients7,9.
With regard to future plastic surgery, the patient should be advised about the likelihood of recurrence of PG, and this point should be explained in an Informed Consent Form to be signed by the patient. Long-term follow-up with a rheumatologist is recommended, as other autoimmune disorders may be found7,9,12.
COLLABORATIONS
|
FFGO |
Conception and design of the study; completion of surgeries and/or experiments; writing the manuscript or critical review of its contents. |
|
MF |
Completion of surgeries and/or experiments. |
|
AMNG |
Completion of surgeries and/or experiments. |
|
OSF |
Completion of surgeries and/or experiments. |
|
MRM |
Completion of surgeries and/or experiments. |
|
EGC |
Final approval of the manuscript; conception and design of the study; completion of surgeries and/or experiments. |
|
OS |
Final approval of the manuscript. |
REFERENCES
1. Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165(6):1244-50. PMID: 21824126 DOI: http://dx.doi.org/10.1111/j.1365-2133.2011.10565.x
2. Wollina U. Pyoderma gangrenosum--a review. Orphanet J Rare Dis. 2007;2:19. DOI: http://dx.doi.org/10.1186/1750-1172-2-19
3. Bittencourt Mde J, Soares LF, Lobato LS, Mançano AD, Leandro HS, Fonseca DM. Multiple cavitary pulmonary nodules in association with pyoderma gangrenosum: case report. An Bras Dermatol. 2012;87(2):301-4. DOI: http://dx.doi.org/10.1590/S0365-05962012000200018
4. Suárez-Pérez JA, Herrera-Acosta E, López-Navarro N, Vilchez-Márquez F, Prieto JD, Bosch RJ, et al. Pioderma gangrenoso: Presentación de 15 casos y revisión de la literatura. Actas Dermosifiliogr. 2012;103(2):120-6. DOI: http://dx.doi.org/10.1016/j.ad.2011.04.010
5. Meyer TN. Pioderma Gangrenoso: Grave e Mal Conhecida Complicação da Cicatrização. Rev Bras Cir Plást. 2006;21(2):120-4.
6. Santos M, Talhari C, Rabelo RF, Schettini APM, Chirano CA, Talhari S. Pioderma gangrenoso - apresentação clínica de difícil diagnóstico. An Bras Dermatol. 2011;86(1):153-6. DOI: http://dx.doi.org/10.1590/S0365-05962011000100025
7. Azulay RD, Azulay LA. Dermatologia. 5ª ed. Rio de Janeiro: Guanabara Koogan; 2011.
8. Fraga JCS, Souza VL, Valverde RV, Gamonal A. Pioderma gangrenoso: apresentação atípica. An Bras dermatol. 2006;81(5 Supl 3):S305-8. DOI: http://dx.doi.org/10.1590/S0365-05962006000900012
9. Souza CS, Chiossi MPV, Takada MH, Foss NT, Roselino AMF. Pioderma gangrenoso: casuística e revisão de aspectos clínico-laboratoriais e terapêuticos. An Bras Dermatol. 1999;74(5):465-72.
10. Konopha CL, Padulla GA, Ortiz MP, Beck AK, Bitencourt MR, Dalcin DC. Pioderma Gangrenoso: um Artigo de Revisão. J Vasc Bras. 2013;12(1):25-33. DOI: http://dx.doi.org/10.1590/S1677-54492013000100006
11. Coelho LF, Correia FG, Ottoni FA, Santos FPST, Pereira LB, Lanna CCD. Pioderma gangrenoso: um desafio para o reumatologista. Rev Bras Reumatol. 2009;49(3):315-20. DOI: http://dx.doi.org/10.1590/S0482-50042009000300013
12. Graças AM, Alecrim ES, Lyon S. Pioderma gangrenoso: evidências clínicas e características. Rev Med Minas Gerais. 2016;26:e-1790.
1. Hospital São Lucas, Serviço de Cirurgia
Plástica Osvaldo Saldanha, Santos, SP, Brazil.
2. Sociedade Brasileira de Cirurgia Plástica, São
Paulo, SP, Brazil.
Corresponding author: Francisco Felipe Góis de Oliveira, Av. Ana Costa, 146 conjunto 1201 - Vila Matias - Santos, SP, Brazil. Zip Code 11060-000. E-mail: felipegoismd@gmail.com
Article received: October 19, 2017.
Article accepted: September 5, 2018.
Conflicts of interest: none.





 Read in Portuguese
Read in Portuguese
 Read in English
Read in English
 PDF PT
PDF PT
 Print
Print
 Send this article by email
Send this article by email
 How to Cite
How to Cite
 Mendeley
Mendeley
 Pocket
Pocket
 Twitter
Twitter